Abstract
Background
Selenium (Se) is an essential element for mammals and its deficiency in the diet is a global problem. Plants accumulate Se and thus represent a major source of Se to consumers. Agronomic biofortification intends to enrich crops with Se in order to secure its adequate supply by people.
Scope
The goal of this review is to report the present knowledge of the distribution and processes of Se in soil and at the plant-soil interface, and of Se behaviour inside the plant in terms of biofortification. It aims to unravel the Se metabolic pathways that affect the nutritional value of edible plant products, various Se biofortification strategies in challenging environments, as well as the impact of Se-enriched food on human health.
Conclusions
Agronomic biofortification and breeding are prevalent strategies for battling Se deficiency. Future research addresses nanosized Se biofortification, crop enrichment with multiple micronutrients, microbial-integrated agronomic biofortification, and optimization of Se biofortification in adverse conditions. Biofortified food of superior nutritional quality may be created, enriched with healthy Se-compounds, as well as several other valuable phytochemicals. Whether such a food source might be used as nutritional intervention for recently emerged coronavirus infections is a relevant question that deserves investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is a semi-metallic element that possesses chemical and physical characteristics of both non-metals and metals (Boyd, 2011). It is recognized as an indispensable micronutrient for redox biology of many animals and unicellular organisms (Kieliszek 2019). In mammals, Se in the form of the unusual amino acid selenocysteine (SeCys) is incorporated at the catalytic center of selenoproteins via recoding of the opal UGA codon into a SeCys codon (Mangiapane et al. 2014). In humans, at least 25 selenoproteins play important roles in antioxidative systems, hormone balance, immunity, male fertility, resistance to viral infections and cancer prevention (Harthill 2011; Rayman 2012, 2020; Tan et al. 2018; Valea and Georgescu 2018). However, Se exhibits a double-edged behaviour, because it becomes toxic over a threshold concentration established to be 400 µg of Se per day (Institute of medicine 2000; Vinceti et al. 2018). For this reason, similar to boron (B), it has long been termed “an essential poison”. Selenium has a relatively narrow safety range as compared to other essential trace nutrients, and thus both the deficiency and toxicity of Se are of worldwide concern (Natasha et al. 2018; Rayman 2020). On the global scale, the Se deficiency and its negative impact on health have progressively increased over recent decades. So far, it has been estimated that 1 billion people have a scanty Se dietary intake (Combs 2001; Tan et al. 2002). This worrisome scenario is likely to worsen in the future because of projected climate changes that will cause reduction of soil Se content, principally in agricultural areas (Jones et al. 2017). Local populations living in low Se areas and feeding on low Se food crops might suffer from Se deficiency disorders because their dietary Se consumption does not meet the daily dose (55–200 µg for adults) recommended to maintain a correct functioning of the metabolism and expression of selenoproteins (USDA–ARS 2012; WHO 2009).
To reduce Se deficiency throughout susceptible regions, the development of high Se crops using biofortification interventions has been accomplished in several countries that are low in Se, such as Finland, United Kingdom, New Zealand, Malawi, parts of China, Tibet, and Brazil (Alfthan et al. 2015; Broadley et al. 2006; Dos Reis et al. 2017; Joy et al. 2019; Wu et al. 2015). Se-biofortified food crops are often enriched in many phytochemicals beneficial to human health, like minerals and antioxidant compounds, which make these foods superior to Se supplementation alone via tablets (D’Amato et al. 2020).
The success of biofortification to enrich plants with Se depends on several variables such as Se species to be used, the mode of Se fertilization, and the crop species. In higher plants, no essential metabolic role of Se has been established and hitherto no specific mechanisms of SeCys incorporation into proteins have been documented (Schiavon and Pilon-Smits 2017a). Selenium is assimilated to Se-amino acids (SeCys and selenomethionine, SeMet) by accessing the metabolic pathway of its analog sulphur (S) (White 2016, 2018). Se-amino acid insertion into proteins in place of S amino acids (cysteine and methionine) can produce malformed proteins causing toxicity (Sabbagh and Van Hoewyk 2012; Van Hoewyk 2013). On the other hand, at low concentration Se can be beneficial to plants. Its positive effects were originally described in Se-hyperaccumulator species (Pilon-Smits et al. 2009). However, further studies with several non-hyperaccumulators have proved that Se promotes the growth, accumulation of beneficial phytochemicals, and antioxidant activity (e.g., Chauhan et al. 2019; Dall’Acqua et al. 2019; Natasha et al. 2018; Schiavon et al. 2013, 2016; D’Amato et al. 2020).
Different approaches can be explored to enrich plants with Se. Some are already widespread or are gaining recognition, while others are limited by in-situ restrictions. The aim of this review is to afford a comprehensive overview of the current knowledge of Se biofortification and future research directions. It is focusing on (i) Se distribution in soil and soil properties that may affect the efficacy of Se biofortification strategies; (ii) Se metabolism in plants and possible interferences of Se enrichment with metabolic pathways that might influence the nutritional profile of Se-enriched food; (iii) Se biofortification strategies (conventional and modern agronomic approaches, genetic engineering and plant breeding) and biofortification under challenging conditions; (iv) effects of Se biofortification on plant food nutritional traits; (v) impact of Se-enriched food on human health with special attention to its role in reducing the risk of and treating viral diseases.
Selenium sources and processes at the plant-soil interface
The geographical distribution of soil Se is very heterogeneous, but Se content in most soils is low and ranges from 0.01 to 2 mg kg− 1 on average (world mean 0.4 mg·kg− 1) (Fordyce 2013; Oldfield 2002). In tropical soils, soil Se levels are slightly higher and typically range within 2–4.5 mg kg− 1 (Mehdi et al. 2013), while seleniferous soils can contain up to 1200 mg Se kg− 1 (Fordyce 2013; Oldfield 2002).
Selenium in soils is primarily derived from the parental material of soil. Its content depends on soil type and texture, origin and geological history, mineralogy, organic matter content, weathering degree and rainfalls, dominant soil geogenic processes, and Se deposition (Hartikainen 2005; Mehdi et al. 2013; Wen and Carignan 2007). Natural Se emissions (crustal weathering, sea spray, volcanic plumes) and anthropogenic Se emissions (fossil fuel combustion, non-ferrous metal production and manufacturing) largely contribute to Se deposition, which is affected by vicinity to the coast, altitude and wind directions (Saha et al. 2017; Wen and Carignan 2007).
Low Se soils mainly derive from igneous rocks and are settled in areas characterized by limited atmospheric depositions and elevate erosion rates (Christophersen et al. 2012). Volcanic soils and granite are commonly poor in Se and lay in the mountainous countries of Northern Europe (Mehdi et al. 2013). Conversely, high Se soils originate from sedimentary rocks, generally Cretaceous sediments like black shales, containing selenites and selenides associated with sulphide minerals. Black shales are particularly abundant in Ireland, China (e.g., Enshi in Hubei Province and Ziyang in Shaanxi Province), and arid areas in the western and southwestern states of the USA (e.g. San Joaquin Valley in California), and India (Punjab) (Oldfield 2002; Winkel et al. 2015). Occasionally, high Se contents in soil may depend on the release of toxic levels of geogenically-derived Se in soils and waters caused by human actions (Ohlendorf et al. 2020), on anthropogenic inputs, either industrial or agricultural, or on dust depositions discharged by nearby coal-burning sites (He et al. 2018). Agricultural activities that increase Se levels in soil include irrigation and the long-term use of chemical fertilizers and farmyard manure. Irrigation may elevate Se in soil by either favoring dissolution of Se from minerals rich in Se or bringing Se loads to soil when Se-rich waters are used (Bajaj et al. 2011), while adding Se to chemical fertilizers is a common practice when biofortification is conducted in Se deficient areas, where soil Se is below 0.6 mg kg− 1, or when prevailing soil conditions hamper Se availability to plants (Gupta and Gupta 2000).
The specific impact and fate of different Se inputs into agricultural soils have been poorly investigated so far. Further systematic mass balance studies of Se in soils that estimate the amount of Se retained in soil and Se losses through leaching, volatilization and crop removal, are required to better quantify the contribute of each Se source to the content of Se in agroecosystems. These studies might be helpful in view of facing with the undergoing global climate-changes predicted to cause reductions of soil Se in the future (Jones et al. 2017).
Selenium in soils is found under inorganic and organic forms, where it holds different oxidation states ranging from − 2 to + 6. Se solubility and mobility in soil increase with increasing oxidizing conditions (high redox potential). In this regard, selenate (SeO42−) is the most water-soluble, mobile and bioavailable inorganic Se species in oxic soils, with low adsorption affinity to oxide surfaces (Hartikainen 2005). Under low soil redox potential conditions, selenate can be reduced to selenite (SeO32−), whose compounds are typically the most abundant Se species in anoxic soils (Fig. 1) (Shahid et al. 2018). Selenite is less mobile and bioavailable than selenate owing to its tendency to be adsorbed on oxide surfaces at low pH (Hartikainen 2005). Therefore, available soil Se is usually poorly correlated with total soil Se. Under intense reducing conditions, selenite can be further reduced to elemental Se (Se0) or selenides (Se2−) (Hartikainen 2005, Winkel et al. 2015). Bacteria are reported to produce (nano)-sized Se(0) from selenate and selenite (Juárez-Maldonado et al. 2019; Ni et al. 2015), while Se in the − 2 oxidation state can exist as hydrogen selenide or metallic selenides, which are very insoluble forms of Se (Winkel et al. 2015).
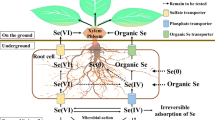
Major pathways of Se at the soil-plant-atmosphere interface, Se movement and partitioning in the plant, with focus on genetic targets for optimization of Se biofortification. Transformation processes in soil are indicated in italics at the corresponding arrows. Soil microorganisms can degrade Se-containing plant litter, thus releasing Se into soil. Se species can be taken up by soil microorganisms or by plants. In the root structure biomagnification, the main transporters involved in Se uptake by plants (SULTR1;2 for selenate, NIP2;1 and PHT2 for selenite, AA Tr. for amino acids) are localized at the rhizodermis (RH). Se metabolism takes place in the root cortical cells (C), and volatile Se species (DMSe and DMDSe) are released into the soil, while Se organic compounds, mainly Se-amino acids (Se a.a.) are transported into the xylem (X) via amino acid permeases (AA Tr.) and delivered to the leaves. DMSe and DMDSe in soil are then de-methylated by rhizosphere microorganisms. Selenate is the main form of Se shuttled through the xylem to the leaves, where it is finally assimilated to Se-amino acids. Selenate is loaded into the xylem by SULTR2;1 localized at the xylem parenchyma cells (XP) and pericycle (P). Both SULTR1;2 and SULTR2;1 from the Se hyperaccumulator S. pinnata are currently under investigation. Organic-Se compounds produced in the leaf cells can be loaded into the phloem (PH) and transported throughout the plant. The translocation of SeMet to the seeds is enhanced by overexpression of the NRT1;1B transporter. Volatile DMSe and DMDSe produced in leaves are released into the atmosphere. Enzymes in circles are early targets of genetic engineering for biofortification or phytoremediation, while enzymes in hexagonal shaped boxes are new targets to better explore. Abbreviations: EN: endodermis; E: epidermis; M: mesophyll; CC: companion cells; CgS: Se Cys γ-synthase. For simplicity, Arabidopsis thaliana has been used as a model species in figure
Generally, soil organic forms of Se occur as complexes with organic matter and combined with organic or organo-mineral colloids (Hartikainen 2005). Organo-Se compounds include methylated or unmethylated Se-amino acids, and volatile Se forms (dimethyl selenide, DMSe, and dimethyl diselenide, DMDSe), but a large fraction of them is still undisclosed (Winkel et al. 2015) (Fig. 1). These compounds can either derive from decomposition processes of plant and microbial biomass or from addition of Se-enriched plant material (green manure) to soil when biofortification of crops with Se is attained.
Several factors control Se mobility and solubility in soils such as pH, sorption, and desorption reactions, redox potential, organic/inorganic compounds, and dissolution processes in soils and sediments. Sorption-interactions depend on the amount of sorption components and are largely governed by electrostatic forces, which in turn rely on soil pH. When Se in soil prevails in anionic form, it can be retain by sorption on oxides and hydroxides (e.g. iron or aluminum oxides/hydroxides), or anionic clays by electrostatic interactions (Eich-Greatorex et al. 2007; Winkel et al. 2015). Se adsorption rate increases while decreasing the pH, as electrostatic interactions become sturdier (Eich-Greatorex et al. 2007). On the other hand, raising in pH or reducing clay and iron (or aluminum) oxide/hydroxide content in the soil result in higher Se mobility and bioavailability.
Soil organic matter (OM) is another critical factor altering soil Se availability, as soils rich in OM commonly display higher Se retention capacity (Fordyce, 2013; Winkel et al. 2015). OM can reduce Se available in soil by direct complexing with Se. Otherwise, indirect complexation of Se with OM-metal complexes or microbial reduction and incorporation into organic compounds (e.g. amino acids, proteins) may occur in soil (Winkel at al. 2015). From either organic complexes or compounds, Se can be promptly mobilized or retained depending on the type of binding.
Selenium uptake and assimilation pathways in plants
Plants accumulate Se in their organs depending on Se phytoavailable in soil, with a large variation of shoot Se concentration between genera, species and even ecotypes within species (Mehdi et al. 2013; Schiavon and Pilon-Smits 2017a; White 2016, 2018).
Plants are distinguished in major ecological groups depending on their capacity to accumulate Se in the shoot when growing in their natural environment (White 2016). Se-hyperaccumulator species thriving on seleniferous soils generally contain and tolerate extremely high Se concentrations (over 1000 µg Se g− 1 d.wt.) that could eventually be toxic to grazers, whereas non-hyperaccumulator species are Se-sensitive and thereby accumulate less than 100 µg Se g− 1 d.wt. (non-accumulators) or up to 1000 µg Se g− 1 d.wt. (accumulators or indicators) (Schiavon and Pilon-Smits 2017a, b; White 2016). Hyperaccumulators store Se mainly in the form of methylselenocysteine (MeSeCys) and selenocystathionine, while SeMet is the main Se organic compound identified in non-hyperaccumulators (Pilon-Smits, 2019; Schiavon and Pilon-Smits 2017a, b).
Plants take up both inorganic (selenate, selenite and elemental Se) and organic (e.g. Se-amino acids) Se species, but not selenides (Chauhan et al. 2019; Gupta and Gupta 2017; Schiavon and Pilon-Smits 2017a; White 2016) or colloidal elemental Se (White 2016, 2018). Their capacity to take up Se is apparently higher for organic over inorganic species (Kikkert et al. 2013). Se-amino acids in particular, are likely to enter the plant cells via broad specificity amino acid transporters (Fig. 1) (Lima et al. 2018). Though, selenate is the main form of Se taken up by plants, and its transport across cell membranes is an energy-dependent process mediated by the sulphate transport system (Lima et al. 2018; Schiavon and Pilon-Smits 2017a; White 2018). Competition events in soil between sulphate and selenate, as well as plant sulphate transporters (SULTR) differing in affinity for these two anions, influence the rate of selenate uptake by plants (El Mehdawi et al. 2018; White et al. 2004, White 2016). Selenite compounds instead, are conveyed over plant cell membranes via phosphorus (P) and silicon (Si) transporters, with differences between selenite anion (SeO32−), hydrogenselenite ion (HSeO3−), and selenous acid (H2SeO3) (Wang et al. 2019; Zhang et al. 2014). Precisely, H2SeO3 is transported by Si transporters (LSI1) and aquaporins (OsNIP2;1) (Wang et al. 2019; Zhao (FJ) et al. 2010, b), while HSeO3− and part of SeO32− mainly use low- and high-affinity P transporters (OsPT2), respectively (Zhang et al. 2014).
Once entered the root cells, inorganic Se is delivered to the plastids where proceeds along the S assimilation pathway to produce SeCys and SeMet (Fig. 2) (Chauhan et al. 2019; Schiavon and Pilon-Smits 2017a; Sors et al. 2005; White 2016, 2018). If selenate is taken up by plants, it must be activated before reduction to selenite. Selenate activation is a rate-limiting step mediated by the ATP sulphurylase enzyme (APS), which couples selenate to ATP to generate adenosine 5′- phosphoselenate (APSe) (Pilon-Smits et al. 2009). APSe reduction to selenite is then conducted by the APS reductase enzyme (APR), via transfer of two electrons from glutathione (GSH). In a parallel pathway, APSe can be phosphorylated to produce 3′-phosphoadenosine 5′-phosphoselenate (PAPSe), which is used as a substrate for sulfation of desulfo-glucosinolates (Fig. 2). Selenite can be reduced enzymatically by the activity of the sulfite reductase enzyme (SiR) to produce selenide, which is incorporated into SeCys by the enzyme complex cysteine synthase, containing both serine acetyl transferase (SAT) and O-acetylserine (thiol) lyase (OAS-TL) enzymes (White 2018). Alternatively, selenite reduction can be realized through a non-enzymatic two step-reaction, where selenite is initially converted to selenodiglutathione (GS-Se-SG) in the presence of GSH. GS-Se-SG is further reduced to selenopersulfide/glutathionylselenol (GS-SeH), which reacts with O-acetylserine (OAS) to generate SeCys (White 2016, 2018). Otherwise, SeCys can be formed directly from selenite by the activity of the selenomethyltransferase enzyme (SMT) (Chauhan et al. 2019) The synthesis of SeMet from SeCys takes place partly in the cytosol and requires selenocystathionine and selenohomocysteine to be formed as intermediates (Fig. 2). This biochemical transformation involves three enzymes working in series: cystathionine γ-synthase (CGS), which promotes the formation of Se-cystathionine by condensing O-phosphohomoserine (OPH) and SeCys; cystathionine β-lyase (CBL) and methionine synthase (MS) (Sors et al. 2005; White 2018).
Schematic diagram of the biochemical reactions of Se assimilation and their subcellular compartmentalization in plants. Enzymes are indicated in italics, major Se compounds and intermediates are indicated in bold. Information was compiled from previous reviews (Chauhan et al. 2019; Schiavon and Pilon-Smits 2017a; Sors et al. 2005; White 2016, 2018). Enzymes: APS: ATP-sulphurylase; APSK: APR: APS reductase; APS kinase; SIR: selenite reductase; CBL: cystathionine β-lyase; SMT: Se cysteine methyltransferase; SeHCysMT: Se-Homocysteine methyltransferase; MT-γ-lyase: methyl Se cysteine-γ-lyase; SL: Se cysteine lyase; MMT: Methionine methyltransferase; OASTL/CS: O-acetylserine thiol-lyase/Cysteine synthase; SOT: sulfotransferase; DMSP lyase: dimethylselenopropionate lyase; Glu-γ-ECS: γ-glutamyl cysteine synthetase. Compounds: GSH/GSSS: reduced/oxidized glutathione; PAPSe: phosphoadenosine 5’- phosphoselenate; OAS: O-acetylserine; Ala: alanine; MeSeMet: Methyl Se methionine; DMSP: dimethylselenopropionate; γ-GluMeSeCys: γ-glutamylmethylselenocysteine; S-MeSeGS: S-Methyl-selenoglutathione; HCys: homocysteine
Incidentally, Se-amino acids can be integrated into proteins, thus disrupting their molecular folding and impairing their function (Sabbagh and Van Hoewyk 2012; Van Hoewyk 2013). The magnitude of this event seems to be more related to the Se/S ratio in plant tissues than the Se content alone (White et al. 2004). To prevent toxicity derived from Se amino acid misincorporation in proteins, SeCys and SeMet can be methylated to produce methyl-SeCys (MeSeCys) or methyl-SeMet (MeSeMet), respectively, which are further converted into volatile DMSe (in non-hyperaccumulators) and DMDSe (in hyperaccumulators) (Pilon-Smits and LeDuc 2009; Van Hoewyk 2013; White 2016). SeCys can also be converted to elemental Se (Se0) and alanine via a reaction ruled by the enzyme SeCys-lyase (SL) (Pilon-Smits and LeDuc 2009), while MeSeCys could be conjugated with glutamate by the enzyme γ-glutamylcysteinesynthetase to form γ-glutamylmethyl-SeCys (γ-GluMeSeCys) (Fig. 2) (White 2018).
How can crops be enriched with selenium? Conventional and novel approaches
A deep knowledge of Se biogeochemistry, uptake mechanisms and assimilation by plants is a mandatory prerequisite for Se biofortification of food crops. Biofortification is the process of adding essential micronutrients and other health-promoting compounds to food crops with the aim to improve the nutritional quality of diets ultimately consumed by people (Garg et al. 2018; Jha and Warkentin 2020; Schiavon and Pilon-Smits 2017b; White and Broadley 2009; Wu et al. 2015; Zhao and McGrath 2009). It is an innovative and reasonably easy practice to manage, affordable and effective in the long-term to tackling micronutrient malnutrition (Ros et al. 2016; White and Broadley 2009). Successful outcomes depend on environmental and economical characteristics of local food systems, but also on their acceptance by farmers and populations that feed on biofortified food. To get acceptance, biofortified crops must be staple, high yielding and worthwhile to be adopted by farmers, and most consumers in target populations must consume the biofortified food in amounts enough to measurably improve their nutritional status (Miller and Welch 2013).
Several studies have been conducted to generate Se-enriched functional food and various biofortification interventions have been developed and tentatively optimized using agronomic approaches, genetic engineering and plant breeding (Bañuelos et al. 2017; Cakmak, 2008; D’Amato et al. 2020; Ros et al. 2016; Schiavon and Pilon-Smits 2017b; White 2016; Zhu et al. 2009). The addition of Se compounds to food during its processing (process fortification) carried out by the food industry has also been proposed as an effective practice in alternative to agronomic biofortification performed using Se fertilizers (Haug et al., 2007). Biofortification strategies, summarized in Fig. 3, are influenced by several variables such as method of Se supplementation, Se dose and species, soil Se, cropping systems and tillage practices, environmental and weathering conditions, crop species/variety, plant growth stage and joint application with other micronutrients (Bañuelos et al. 2017; D’Amato et al. 2020; Dall’Acqua et al. 2019; Hawrylak-Nowak, 2013; Schiavon et al. 2013, 2016). So far, most research dealing with Se biofortification has targeted at the application of Se alone or occasionally combined with only one micronutrient, generally silicon, iodine (I) or zinc (Zn) (Golubkina et al. 2018; Sattar et al. 2017; Cakmak et al. 2020; Golob et al. 2020). Only few studies have addressed crop biofortification with multiple micronutrients (Mao et al. 2014; Zou et al. 2019), although deficiency of multiple trace nutrients is an issue of global concern. A recent field experiment managed in six different countries (China, India, Mexico, Pakistan, South Africa, and Turkey) varying in soil type and environmental conditions, proved that foliar fertilization of wheat (Triticum aestivum L.) plants with a blend of Se, Fe, I, and Zn, was effective in enriching grains with all four elements (Zou et al. 2019). Based on these promising results, future Se biofortification research should address the feasibility of supplementing crops with cocktails of micronutrients without unwanted crop yield trade-off.
Overview of biofortification strategies that can be exploited to enrich crops in selenium. Se biofortification can be achieved using genetic tools (1), via conventional or assisted breeding and genetic engineering, or through fertilization with Se-fertilizers via foliar application (2) or soil amendment (3). Agronomic biofortification can be integrated by the use of rhizosphere or endophytic microorganisms, either inoculated into plants (4) or applied to soil (5). Alternatively, plant material enriched with Se-organic compounds from hyperaccumulators or accumulators used in phytoremediation, or from crops grown in naturally enriched soils can be used as green manure (6). Nanosized biofortification is performed using SeNPs applied to leaves or soil (7). Co-cropping or intercropping practices use Se-hyperaccumulators to enrich soil with Se-organic compounds promptly available for uptake by neighboring plants or succeeding crops (8)
Agronomic biofortification
Agronomic biofortification is commonly employed in low Se areas and mainly consists in Se addition to soil and Se foliar fertilization, typically realized using selenate- or selenite-based fertilizers (Alfthan et al. 2015; Broadley et al. 2006; Wu et al. 2015). Selenium fertilizers are generally employed in small amounts (10–20 g Se ha− 1) to achieve biofortification goals. Therefore, to ease their application, they are frequently mixed into other commercial nutrient fertilizers (e.g., mix of nutrients, urea, calcium nitrate) functioning as “carriers” of Se (Premarathna et al. 2012; Ramkissoon et al. 2019). The addition of organic acids to Se fertilizers can further promote Se chelation with organic compounds, thus causing incremental plant uptake of Se and efficiency of Se fertilizers.
The application of Se fertilizers to soil commonly results in increased total and bioavailable Se (Broadley et al. 2010), and thus in higher Se concentration in crop edible products (Chilimba et al. 2012; Curtin et al. 2006; Lyons, 2010; Poblaciones et al. 2014). Such a practice is apparently not associated with environmental risks of Se leaching into groundwater, being this process limited by Se binding to soil organic matter and positively charged sites, and Se losses via volatilization (De Feudis et al. 2019). Field tests have been successfully conducted by applying Se to soil in Finland (Alfthan et al. 2015; Hartikainen, 2005), UK (Broadley et al. 2010; Lyons, 2010), and New Zealand (Curtin et al. 2006). Nevertheless, the efficacy of this approach can be limited by heterogenous distribution of Se in soil and by those soil conditions that control Se speciation and uptake by plants such as pH, organic matter, oxygenation, content of competing ions, soil age, chemical and biological Se transformations (Bañuelos et al. 2017; Duncan et al. 2017; Haug et al., 2007; Schiavon and Pilon-Smits, 2017b; Stroud et al. 2010). It has been estimated that only 12% of soil-applied Se fertilizers is taken up by plants on average, as most of Se is fixed and retained in the soil, and thus is not bioavailable (Broadley et al. 2010). Negligible residual Se is then available for succeeding crops, and thus Se fertilizers must be applied to soil for each growth period. In contrast, foliar Se fertilization is up to 8 times more effective than soil Se supplementation (Ros et al. 2016). This is likely because of more rapid Se uptake and assimilation processes, no need of Se root-to-shoot translocation to the edible parts of crops, and prevention of Se losses due to immobilization of Se compounds in soil (Ramkissoon et al. 2019).
However, soil amendment with Se can determine positive shifts in the amount and diversity of soil microbial communities, by increasing the relative abundance of plant growth promoting rhizobacteria (PGPR) and reducing the occurrence of pathogenic fungi (Liu et al. 2019). Beneficial rhizosphere microorganisms could enhance soil Se phytoavailability and the plant Se use efficiency of applied fertilizers (Durán et al. 2014; Lindblom et al. 2014; Mora et al. 2015; Yasin et al. 2015a,b) owing to their capacity to reduce oxidized and methylated forms of Se (Winkel et al. 2015) and increase the volume of soil explored by the plant roots to acquire Se (White and Broadley 2009). The addition of beneficial microorganisms to soil or the inoculation of plants with PGPR might enhance Se biofortification of crops. For instance, the inoculation of wheat plants with specific microorganisms, alone or combined with arbuscular mycorrhizal fungi (AFM), was effective in increasing Se concentration in their edible products (Duran et al. 2013; Yasin et al. 2015a,b). Similarly, inoculating shallot bulbs with AFM increased selenocystine and selenate contents by 36% and 21%, respectively, compared to non-inoculated plants (Golubkina et al. 2019).
Crop enrichment with Se could also be achieved by combining different types of Se phytotechnologies (Bañuelos et al. 2015; Schiavon and Pilon-Smits 2017b; Stonehouse et al. 2020). Se-laden plant material derived from plants employed in Se phytoremediation could be recycled as green fertilizer to increase Se content in agricultural soils or as a supplemental fodder for livestock (Bañuelos et al. 2009). In this case, before being introduced into the food web, the plant material must be carefully checked for its content in hazardous elements, as soils intended to be remediated are often contaminated with many metal/loids. Alternatively, plant material derived from Se-hyperaccumulators thriving on seleniferous soils and containing high levels of Se, especially in organic forms (e.g. SeMet, MetSeCys), could be used as green manure (Bañuelos et al. 2015, 2017; Wan et al. 2018). Crops to be amended with this material may be selected via breeding or engineered for preferential uptake and accumulation of organic Se in edible products. Such an approach might have relevant implications for healthy nutrition, because organic Se compounds in food may be more quickly used than inorganic Se by enzymes that support antioxidant activities in humans and animals (Davis 2012). Se-hyperaccumulators could be otherwise used in co-cropping or intercropping practices. In this case, they will deposit their leaf litter on soil at the end of their growing season, and thus the soil will be enriched with Se organic compounds available for the uptake by the neighboring crops.
Growing crops is soils naturally rich in Se or irrigated with waters naturally abundant in Se could be another Se biofortification option to explore. This practice is known as “natural biofortification” and is gaining interest in certain areas worldwide (Wu et al. 2015). It has been realized in some regions of China (Dinh et al. 2018), USA (Bañuelos et al. 2015, 2019) and India (Dhillon and Dhillon 2009), but the number of studies conducted in the field environment is still limited so far, as most research has been performed under controlled and simplified environments, with homogeneous soil properties that do not resemble the real soil conditions of agroecosystems. One drawback of this practice is that agricultural use of seleniferous soils can hasten the release of Se in the environment under certain conditions, particularly when the Se gets concentrated by evapotranspiration, thus generating possible eco-toxicological hazard.
Biofortification by using nano-sized selenium
In very recent years, emerging cutting-edge technologies have advised the application of Se in the form of Se nanoparticles (SeNPs) in alternative to conventional Se fertilizers for enriching crops with Se-organic compounds (Babajani et al. 2019; Juárez-Maldonado et al., 2019). SeNPs vary in shape and size and can be synthesized from Se salts precursors, mainly selenite and selenate, in the presence of reducing agents (e.g. proteins, phenols, alcohols and amines) produced by bacteria, fungi and plant extracts (Husen and Siddiqi 2014; Nayantara and Kaur 2018). The biogenic conversion of Se salts to zerovalent SeNPs by Se specialist bacteria has been described in a number of studies (Hunter and Manter 2008; Hunter 2014; Ni et al. 2015), but a great task of scientists remains the synthesis of clonable SeNPs. Indeed, the effects of nanoparticles (NPs) on plants, beyond depending on NP concentration and mode of application (foliar, substrate, seeds), are strongly affected by the method handled for their synthesis, which is further responsible for NP specific properties (size, shape). Recently, Pseudomonas moraviensis stanleyae, a bacterium isolated from the roots of the Se hyperaccumulator Stanleya pinnata, showed to tolerate extremely high Se concentrations and produce intracellular SeNPs, and its glutathione reductase enzyme has been proposed as a good candidate for the synthesis of clonable SeNPs (Ni et al. 2015).
Harnessing SeNPs as Se-nanofertilizers holds the potential for synchronized Se management in terms of release and uptake by crops, while preventing Se losses in agroecosystems that may occur when commercial Se fertilizers containing selenate and selenite salts are employed (Babajani et al. 2019). SeNPs seem to be less toxic to plants than selenate and selenite salts, as reported in tobacco (Nicotiana tabacum L.) (Domokos-Szabolcsy et al. 2012), garlic (Allium sativum L.) (Li et al. 2020) and Vigna radiata plants (Bărbieru et al. 2019). They can also improve the quality traits of vegetables, as described in strawberry (Fragaria × ananassa) and tomato (Solanum Lycopersicon L.), whose fruits were more enriched in organic acids (e.g., malic, citric and succinic acids) and sugars (e.g. glucose, fructose and sucrose) after treatment with SeNPs (Morales-Espinoza et al. 2019; Zahedi et al. 2019). However, SeNPs may induce toxicity at high levels (Hussein et al. 2019). For instance, in groundnut (Arachis hypogaea L.) plants, low doses of SeNPs improved yield components and seed oil, but high doses altered the protein profile and fatty acid composition by increasing unsaturated fatty acids and/or decreasing saturated fatty acids compared to untreated plants (Hussein et al. 2019).
Despite the advantages offered by Se-agronanotechnologies, insufficient literature exists on SeNPs action in plants and their interactions with plant ecological partners, transformation in the food web and the environment. SeNPs are largely used in pharmaceutical applications to increase the bioavailability of drugs and targeting therapeutic agents to specific organs, and are envisioned as next-generation safe ingredients for dietary supplements because they can gradually release Se and better control targeted mechanisms of actions, with only few side effects (Constantinescu-Aruxandei et al. 2018). Though, the release of SeNPs and their fate in the environment following biofortification may pose concerns, cause the lack of information about the impact of SeNPs on public safety and their potential toxicity. It must be noted that SeNPs are becoming emerging contaminants in some areas worldwide due to their intense use in electronics and material productions (Rashid et al. 2016). Therefore, to avoid any unpredictable health hazard from SeNPs occurrence in the environment, further studies should systematically address SeNPs risk assessment and management when used to agricultural purposes.
Biofortification using plant genetics
Selenium biofortification through conventional breeding is likely to be the most established, sustainable and long-term method of crop biofortification (White and Broadley 2009). It is termed “genetic biofortification” and aims at selecting plant cultivars of high/moderate capacity to take up and translocate Se to the edible parts, or having preferential uptake of organic Se (SeMet and/or MetSeCys) (Bañuelos et al. 2017; Broadley et al. 2006; Wu et al. 2015; Zhu et al. 2009). Breeding of crops with high ability to accumulate Se is a feasible practice because enough inter- and intraspecies genetic variation exists in grain Se concentration of several cereal and leguminous crops, as well as in onion bulbs (Allium cepa L.), Brassicaceae spp., chicory (Cichorium intybus L.), lettuce (Lactuca sativa L), tomato (Solanum lycopersicum L.) and pepper (Capsicum annuum L.) fruits, and potato (Solanum tuberosum L.) tubers (for specific references see review from White 2016). In this regard, it has been speculated that soil Se phytoavailability and environmental factors are primary factors in controlling Se concentration in edible parts, especially in cereal grains, and that genotypic variation of Se concentration might become more relevant under high Se phytoavailability (Zhu et al. 2009).
So far, a number of chromosomal loci (QTLs) associated with high Se accumulation in grains and leaves of several crops have been identified (Ates et al. 2016; Norton et al. 2010; Wang et al. 2017; White 2016; Zhang et al. 2006). Selecting plant cultivars of high Se concentration in the edible products can be used in marker-assisted breeding (MAB) to transfer these high-Se QTLs to high-yielding low Se cultivars (Pilon-Smits and LeDuc 2009; Wu et al. 2015). One main limitation in the plant breeding, either conventional or marked assisted, is that it must be integrated by agronomic biofortification interventions using Se fertilizers when crops are grown in low Se areas.
The advent of modern molecular tools and analytical technologies has brought to advances in research focusing on Se biofortification for designing future and more effective strategies. Analytical methods include synchrotron X-ray fluorescence and X-ray absorption near edge structure spectroscopies principally, while molecular technologies benefit from high-speed and low-cost of next generation sequencing (NGS), and encompass oligo-directed mutagenesis, reverse breeding, RNA-directed DNA methylation, and DNA editing (Carvalho and Vasconcelos 2013). Such technologies, along with functional genomics gene technology, might be supplementary tools to breeding and genetic engineering (Hung et al. 2012; Pilon-Smits and LeDuc 2009; Wang et al. 2018). However, genetic engineering is far from being widespread and accepted compared to agronomic biofortification and conventional breeding because of imperative restrictions in the use of transgenics yet existing in many countries (Zhu et al. 2009).
A few transgenics suitable for biofortification have been generated so far, with increased capacity to acquire and accumulate Se, preferentially in organic form, in the edible products (Pilon-Smits and LeDuc 2009; White and Broadley 2009; Zhu et al. 2009). Most of these transgenics overexpress sulphate transporters, or enzymes that catalyze rate-limiting steps in Se assimilation such as ATP-sulphurylase, or are involved in mechanisms that prevent Se misincorporation into proteins, like selenocysteine lyase and selenocysteine methyltransferase (Zhu et al. 2009).
Other genes have been successfully targeted by genetic engineering in the last decade, with positive outcomes for Se biofortification. For instance, the overexpression of the selenium binding protein gene SBP1 in Arabidopsis thaliana enhanced the resistance of plants to selenite via a GSH-dependent mechanism (Agalou et al. 2005). Similarly, the loss-of‐function mutations in the gene APX1 coding for a cytosolic ascorbate peroxidase enzyme or the overexpression of the Ethylene response factor ERF96 improved Se tolerance and accumulation in A. thaliana (Jiang et al. 20162020). In the Se accumulator Brassica juncea L., a novel selenocysteine methyltransferase enzyme has been identified, which is capable to methylate both homocysteine and SeCys substrates (Chen et al. 2019). The overexpression of this enzyme in tobacco plants increased total Se and MeSeCys accumulation (Chen et al. 2019). Another potential gene target is the NRT1.1B transporter, a member of the rice peptide transporter (PTR) family involved in nitrate transport, because it displays SeMet transport activity and its overexpression in rice leads to higher SeMet accumulation in grains (Zhang et al. 2019).
New targets for genetic manipulation have also been identified in the Se-hyperaccumulator Stanleya pinnata, such as selenate/sulphate transporters (El Mehdawi et al. 2018) and one isoform of ATP-sulphurylase (APS2) (Jiang et al. 2018). S. pinnata owns a root high affinity sulphate/selenate transporter, SULTR1;2, which is constitutively highly expressed and does not undergo the canonical repression operated by high sulphate in non-hyperaccumulators (El Mehdawi et al. 2018; Wang et al. 2018). In addition, this hyperaccumulator shows elevate expression of the low affinity sulphate transporter SULTR2;1, which plays a role in sulphate/selenate root to shoot shuttling. Both SULTR transporters could be potential candidates of transgenic technologies for the generation of high Se crops. The APS2 isoform of S. pinnata instead, is a quite unique and fascinating enzyme, as its expression exclusively localizes to the cytosol (Jiang et al. 2018). Normally, APS isoforms work in the plastids for S/Se assimilation and the APS2 isoform from two Se non-hyperaccumulators, A. thaliana and Stanleya elata, has a dual localization, plastidial and cytosolic (Bohrer et al. 2015; Jiang et al. 2018). The elevate expression of APS2 in S. pinnata might be responsible for its Se hypertolerance. For this reason, APS2 function is currently under investigation.
Early and novel targets of genetic engineering are indicated in Fig. 1.
Selenium biofortification in the context of global environmental challenges
Plants often face with adverse environmental constraints, which are mostly the result of anthropogenic activities and global climate changes (Mall et al. 2017). Soil salinity, drought and heat stress in particular, have a negative impact on sustainable agricultural systems and severely impair crop yields, especially in arid and semi-arid regions where rainfalls are scarce. Plants growing under such conditions suffer from osmotic stress along with oxidative stress due to increased production of reactive oxygen species (ROS) (Fahad et al. 2017). In this regard, stressed crops might get benefits from Se biofortification, Se acting as a direct or indirect antioxidant (Natasha et al. 2018).
Overall, the beneficial effects triggered by Se are primarily associated to its dose and the capacity of plants to accumulate and tolerate Se (Hawrylak-Nowak 2013; Ríos et al. 2010; Schiavon et al. 2013). At low doses Se promotes the reduction in electrolytic leakage and the recovery of cell integrity under various stress conditions (Zembala et al. 2010; Malik et al. 2012), and enhances the cell antioxidative capacity by stimulating the activity of antioxidant enzymes (superoxide dismutase, SOD, catalase, CAT, peroxidase, POD, ascorbate peroxidase, APX, monodehydroascorbate reductase, MDHAR, dehydroascorbate reductase, DHAR, glutathione peroxidase, GPX, glutathione reductase, GR, and glutathione-S-transferase, GST) and the synthesis of antioxidant metabolites (e.g., ascorbic acid, AsA, glutathione, GSH), as a mechanism for mitigating the effects of ROS (Hasanuzzaman and Fujita 2011; Hasanuzzaman et al. 2011; Jiang et al. 2017; Pilon-Smits et al. 2009). Indeed, at low levels ROS mediate the signal transmission in the defense responses of cells under stress, but at high levels they injure cell components. Therefore, the regulation of the antioxidant system by Se may lead to the reduction of ROS and, in this way, changes their role from detrimental to beneficial. In addition, low Se has been reported to delay plant senescence (Hajiboland et al. 2019), which can be induced prematurely by stress conditions causing reduction of crop yields. In this respect, Se modulates the levels of nitric oxide (NO) in plants (Hajiboland et al. 2019), which in turn negatively regulates several elements (receptors, signal transduction proteins and/or transcription factors) involved in ethylene production and senescence processes (Freschi 2013).
Nevertheless, at high doses Se provokes the synthesis of malformed proteins, reacts with thiol groups and GSH to induce ROS over-generation (Gosh and Biswas 2017; Natasha et al. 2018; Van Hoewyk 2013), and disrupts reactive nitrogen species (RNS) resulting in protein tyrosine nitration (Gupta and Gupta 2017; Kolbert et al. 2018). During high Se condition, GSH can be possibly depleted because of Se/S competition for uptake and assimilation, thereby enhancing ROS accumulation (Dall’Acqua et al. 2019; Van Hoewyk 2013).
Typical beneficial effects of Se described in food crops growing in salinized environments generally include the improvement of agronomic traits (e.g. shoot length, shoot diameter, fresh and dry biomass) (Diao et al. 2014; Hawrylak-Nowak 2009; Hasanuzzaman et al. 2011; Jiang et al. 2017), the increase of net photosynthesis and water contents (Diao et al. 2014; Habibi 2017; Hawrylak-Nowak 2009; Jiang et al. 2017), the stimulation of antioxidant processes and accumulation of non-enzymatic antioxidants (AsA GSH, phenolic compounds), accumulation of osmolytes (proline, sugars and K+), the reduction of lipid peroxidation and ROS concentration in cells, and the regulation of Na+ homeostasis through increased expression of genes encoding Na+/H+ antiporters (NHX), as reported in maize (ZmNHX1) and rice (OsNHX1), which results in enhanced sequestration of Na+ in the root vacuoles to prevent Na+ delivery to the shoot (Ashraf et al. 2018; Elkelish et al. 2019; Habibi 2017; Hasanuzzaman et al. 2011; Hawrylak-Nowak 2009; Kamran et al. 2019; Jiang et al. 2017; Subramanyam et al. 2019). Under certain circumstances, the positive action of Se in salt stress physiology could be intensified by giving this element along with silicon (Si) (Sattar et al. 2017).
In plants suffering from drought stress or heat stress, Se fertilization may sustain crop yields by alleviating ROS-induced damages via activation of cellular antioxidant systems (D’Amato et al. 2018a; Iqbal et al. 2015; Jóźwiak and Politycka 2019; Malerba and Cerana 2018; Sajedi et al. 2011). Increased activity of antioxidant enzymes and reduced lipid peroxidation have been reported in wheat, maize and cucumber plants grown under water deficit (Nawaz et al. 2016; Yao et al. 2009) and in wheat plants subjected to heat stress (Iqbal et al. 2015; Jóźwiak and Politycka 2019). Foliar Se application increased the relative water content, pigment and free amino acid accumulation, and the agronomic traits (e.g. fodder yield, crude protein, fiber, nitrogen free extract) of water-stressed maize plants (Nawaz et al. 2016), and promoted gas exchanges, Fe uptake and accumulation of osmoprotectants (total soluble sugars and free amino acids) in wheat, thus ultimately increasing grain yield (Nawaz et al. 20152016). Recently, high accumulation of osmolytes like proline and K+, and intense N metabolism have been described in maize plants cultivated in soil fertilized with Se and poorly water-irrigated (Bocchini et al. 2018). Epigenetic changes in DNA methylation in these plants caused by simultaneous water deficit and Se fertilization indicated that Se could up-regulate the expression of a number of genes implied in the plant tolerance to environmental stresses, like those coding for phytoene synthase (PSY), important enzyme for the preservation of cell carotenoids, sorbitol dehydrogenase (SDH), which controls osmolyte biosynthesis under drought stress, and alcohol dehydrogenase (ADH), functioning in plant adaptation to abiotic stress.
Selenium biofortification is also efficient in ameliorating the nutritional quality of vegetable products derived from plants grown under drought stress. For instance, olives produced by olive trees subjected to water shortage hold higher fresh weight and increased the level of phenols and pigments at harvest (D’Amato et al. 2018a). Furthermore, the extra virgin olive oil (EVOO) obtained from these olives was of superior nutritional value due to the longer shelf life and improved oxidative stability against oxidation processes.
In addition to the positive effects elicited by Se in plants subjected to abiotic stress, Se supplementation has been reported to protect plants from biotic stress caused by pathogenic fungi (Kornas et al. 2019; Xu et al. 2020) and a variety of generalist invertebrates and herbivores (Freeman et al. 20072009; Quinn et al. 2010; Valdez Barillas et al. 2011), suggesting that crops enriched with Se may require less synthetic fungicides and pesticides during their cultivation. Therefore, besides being applied to crops to address malnutrition in vulnerable populations, Se might be used to attain agro-ecological plant protection and sustainable use of agrochemicals. Alternatively, Se could be employed as an ecological insecticide (Mechora 2019) or fungicide (Wu et al. 2014; Xu et al. 2020). In this respect, Wu et al. (2014) proposed the use of Se in soil for the control of the postharvest disease of fruits and vegetables caused by Penicillium expansum, while Liu et al. (2019) and Xu et al. (2020) endorsed the use of Se for the control of the oilseed rape disease caused by Sclerotinia sclerotiorum.
Impact of Se biofortification on food crop nutritional traits
Effects of Se-biofortification on accumulation of Se species
1Beyond achieving increases of total Se concentration in crops, one main task of Se biofortification is to enrich plants with Se compounds that provide health benefits to humans and animals (Fig. 4) (Newman et al. 2019). SeCys and SeMet are important precursors for the synthesis of mammalian selenoproteins, function as direct antioxidants in cells due to their easy oxidation and have a major role in protein repair (Rahmanto and Davies 2012). Furthermore, SeMet and other organic Se compounds like methylseleninic acid (MSA), methylselenol (MSe), γ-glutamyl-selenium-methylselenocysteine (γ-GluMeSeCys) and MeSeCys, have recognized efficacy in chemoprevention (Spallholz 2019; Tarrado-Castellarnau et al. 2015; Vinceti et al. 2018).
Metabolic targets of Se biofortification in terms of Se species (1a-d), glucosinolates (2, only for Brassicaceae spp.), other nutrients and beneficial phytochemicals (3a-d). Plants have been divided in 4 categories according to D’Amato et al. (2020): cereal crops, vegetables, microgreens and fruit trees
Crop species and varieties differ in the ability to convert inorganic Se into organic forms. Cereal crops in particular, can store Se in seeds mainly in the form of SeMet (Broadley et al. 2010), whose translocation in rice is apparently promoted by the NRT1.1B transporter (Zhang et al. 2019). Brassicaceae spp., including broccoli (Brassica oleracea L.), radish, Chinese cabbage (Brassica rapa L.), salad rocket and wild rocket can accumulate high amounts of organic Se species, especially SeMet and/or MeSeCys (Bachiega et al. 2016; Bañuelos et al. 2015; Ramos et al. 2011; Schiavon et al. 2016; Šindelaŕǒva et al. 2015). The same Se compounds have been identified in hemp (Cannabis sativa L.), rice (Oryza sativa L.), and potato (Solanum tuberosum L.) (Huang et al. 2018; Stonehouse et al. 2020; Zhang et al. 2019). Leguminous plants spp. like chickpea (Cicer arietinum L.), soybean (Glicine max L.), and alfalfa (Medicago sativa L.) accumulate SeMet and SeCys, but not methylated Se amino acids (Chan et al. 2010; Hajiboland and Amjad 2008; Poblaciones et al. 2014). Organic Se has been found in olive trees (Olea europaea L.), and pear trees (Pyrus communis L.), but the Se species have not been determined (D’Amato et al. 2018a; Deng et al. 2019). Leafy vegetables like lettuce (Lactuca sativa L.), basil (Ocimum basilicum L.) and Cichorium spp., instead, contain only inorganic Se (Hawrylak-Nowak 2008; Stibilj et al. 2011).
Effects of Se-biofortification on Se-S crosstalk and accumulation of S-metabolites
One common observation from biofortification studies is that the content of inorganic or organic Se species tendentially builds up in crops fertilized with Se, while the amount of S-containing phytochemicals with functional roles in plant defense mechanisms and human health, such as glucosinolates (GLS), glutathione and S amino acids, might undergo variation depending on Se dose and species to be used, plant species and genotype, and mode of Se fertilization (foliar fertilization or soil amendment with Se) (Bachiega et al. 2016; Robbins et al. 2005; Schiavon et al. 2013; Schiavon and Pilon-Smits 2017b). GLS and their hydrolysis products (isothiocyanates, ITC) in particular, are recognized key molecules in cancer prevention, and have received great attention in biofortification studies of Brassicaceae spp. (Melrose 2019; Prieto et al. 2019). Increased accumulation of ITC has been recently observed in kale (Brassica oleracea L.) roots in response to selenite and NaCl joint application, thus making this food crop superior for chemopreventive effects (Kim et al. 2018).
The impairment of S metabolites has been frequently observed in plants supplemented with high selenate or selenite doses. This is likely because Se and S compete for the uptake and metabolic processes in plants (McKenzie et al. 2019; Schiavon and Pilon-Smits 2017a; Tian et al. 20162018). Negative effects of Se/S competition on the synthesis of GSH and GLS precursor amino acids, and on the expression of genes within the GLS pathway have been largely described (Dall’Acqua et al. 2019; McKenzie et al. 2019; Schiavon et al. 2016; Tian et al. 2016). Methionine (Met) is the precursor amino acid for the synthesis of aliphatic GLS, and the decrease of its content in response to Se fertilization has been associated with lower accumulation of Met-derived-GLS in salad rocket (Eruca sativa Mill.), radish (Raphanus sativus L.) and other Brassicaceae spp. (Dall’Acqua et al. 2019; Schiavon et al. 2016). In broccoli, discrepant results have been reported. Barickman et al. (2013) and Robbins et al. (2005) noted the decline of aliphatic GLS and sulforaphane, a GLS hydrolysis sulphur-containing aglycon byproduct, in broccoli supplied with high Se. In contrast, Sepúlveda et al. (2013) did not find any significant change in GLS and sulforaphane content, neither in myrosinase activity, in broccoli treated with high Se (100 µM selenate). Similarly, Tian et al. (2016) did not measure any appreciable variation of GLS content in broccoli sprouts exposed to 100 µM selenite or selenate, and hypothesized an equilibrium between GLS biosynthesis and hydrolysis.
Differential effects of Se application on the synthesis of S-metabolites can be observed between distinct plant species of the same genus, as recently reported in two rocket species, namely salad rocket and wild rocket (Diplotaxis tenuifolia) (Dall’Acqua et al. 2019). Rocket salad plants treated with 20 and 40 µM selenate exhibited reduced capacity to accumulate S amino acids, GSH and GLS, mainly because of Se/S competition processes and repression of genes involved in S reduction and GLS synthesis and hydrolysis. In contrast, wild rocket plants supplied with equal selenate concentrations accumulated more Se, without significant effects on S uptake and metabolism. Interestingly, wild rocket contained large levels of osmolytes (proline) in response to increasing Se application, likely to prevent the oxidative stress that is often generated by high Se in tissues.
Beside S-glucosinolates, Brassicaceae spp. supplemented with Se can produce (methylseleno)glucosinolates and their Se-containing aglycons (Matich et al. 20122015; McKenzie et al. 2019), whose bioactivity as anticancer and antimicrobial agents is considered higher compared to GLS (Emmert et al. 2010). The majority of Se-GSL identified so far are aliphatic, with Se in the position of the S donated by Met and not in the sulphate group or on the bond with glucose (Matich et al. 20122015). The Se-glucosinolates 3-(methylseleno)propyl glucosinolate, 4-(methylseleno)butyl glucosinolate, 5-(methylseleno)pentyl glucosinolate, 4-(methylseleninyl)butyl glucosinolate and seleno-2-phenylethyl glucosinolate have been described in broccoli, cauliflower and forage rape (Matich et al. 20122015), while 4-(methylseleno)but-3-enyl glucosinolate and related isothiocyanates (isomers of 4-(methylseleno)but-3-enyl isothiocyanate) have been tentatively identified in radish plants (McKenzie et al. 2019). In Se-enriched radish and broccoli, the up-regulation of the gene coding for APS kinase (APSK), the key branch point enzyme of GSL biosynthesis, was observed and explained as a protective mechanism activated by plants to control S uptake and limit Se-GLS production (McKenzie et al. 20172019). The repression of genes encoding enzymes promoting aliphatic GLS biosynthesis and the concomitant up-regulation of genes coding for enzymes involved in indole GLS generation were also hypothesized as part of the same protective mechanism that aims to generate indole GLSs over aliphatic GLS to restrict the synthesis of Se-GLS (McKenzie et al. 2019).
Effects of Se-biofortification on accumulation of other health beneficial phytochemicals
At low doses Se is beneficial to plants and typically improves the antioxidative capacity, increases the content of nutrient elements and improves the profile of worthwhile phytochemicals. Thus, high Se food represents a valuable tool to improve the diet, nutritional quality and health in world population. Very recently, D’Amato et al. (2020) surveyed the impact of Se biofortification on the nutraceutical profile of food crops. The authors grouped them by crop category (arable crops, vegetables, microscale vegetables, and fruit trees) and highlighted the foremost effects of Se biofortification on the content of total Se, inorganic/organic Se species, and bioactive compounds, which are summarized and implemented in Fig. 4.
With respect to arable crops, the enrichment with Se mostly leads to higher antioxidant activity of their grains and greater content of nutrients, amino acids, phenols, anthocyanins, sugars, and organo-Se compounds (D’Amato et al. 201720192020; Skrypnik et al. 2019). Also, in upland rice polished grains, Se application induced higher concentration of storage proteins like albumin, globulin, prolamin, and glutelin (Reis et al. 2020).
In horticultural vegetables, Se fertilization is ascertained to augment the antioxidant activity, nitrogen and S assimilation, accumulation of pigments (carotenoids and anthocyanins), soluble phenols, and ascorbic acid (Dall’Acqua et al. 2019; Hawrylak-Nowak et al. 20082018; Mimmo et al. 2017; Schiavon et al. 20132016; Ríos et al. 2010). Enhanced production of essential oils, hydroxycinnamic acids, total phenolics, anthocyanin and antioxidant activity has been recently reported by Skrypnik et al. (2019) in sweet basil (Ocimum basilicum L.) leaves, while increased accumulation of flavonoid compounds, especially naringenin chalcone and kaempferol, and several amino acids except proline, was observed in Se-biofortified tomato fruits and radish roots, respectively (Schiavon et al. 20132016).
Seedlings of edible vegetables supplemented with selenate seem to preferentially accumulate organic Se, and their nutritional value is generally ameliorated by Se enrichment (D’Amato et al. 2018b2020; Islam et al. 2020; Pannico et al. 2020). In fact, Ávila et al. (2014) screened Se-enriched cultivars from the six largely consumed Brassica vegetables and found that all were able to accumulate MeSeCys and contained higher levels of GLS, especially glucoraphanin. Increased accumulation of several bioactive compounds (carotenoids, phenolics, flavonoids, ascorbic acid, and anthocyanins) was observed in Se-biofortified wheat microgreens (Islam et al. 2020). Similarly, Se fertilization induced larger accumulation of total phenolics in coriander (Coriandrum sativum L.), green basil, purple basil, and tatsoi (Brassica narinosa L.) microgreens grown in soilless cultivation (Pannico et al. 2020), while Se-enriched chickpea sprouts were proved to be a valuable source of oil characterized by high oxidative stability index (OSI) and cellular antioxidant activity (CAA), due to reduced lipase and lipoxygenase (LOX) activities and increased content in phenolics, respectively (Guardado-Félix et al. 2019). Interestingly, Puccinelli et al. (2019) suggested the production of Se-enriched sprouts from seeds collected from plants fertilized with Se as a novel biofortification approach.
Little information is available about the effects of Se on fruit trees (D’Amato et al. 2020). In the few studies accomplished in this area of research, spraying leaves or fruits with Se was the only approach used to enrich fruits (e.g. pears, peach, apples, olives) with Se (D’Amato et al. 20172018ab; Deng et al. 2019; Pezzarossa et al. 2012). Se foliar fertilization of olive trees increased the accumulation of several nutritional components of olives and EVOO, such as mineral elements, pigments (carotenoids and chlorophylls) and phenolic compounds (e.g. oleacein, ligustroside aglycone, and oleocanthal) (D’Amato et al. 20172018a). Such results indicate that Se fertilization might be used to increase the nutritional value of EVOOs in order to meet the quality standards required by the European Food Safety Authority (EFSA) (D’Amato et al. 2020).
Impact of high Se food on emerging viral epidemics
Selenium deficiency in humans is associated with a variety of pathological conditions, including a cardiomyopathy endemic in China termed Keshan disease, reduced male fertility, mood disorders, and disturbance of thyroid functions (Rayman 20122020). The Se nutritional status of individuals also impacts on the immune system defenses activated to combat viral infections and on the viral pathogen itself (Guillin et al. 2019; Harthill 2011). If Se level in blood is below 1µM, the metabolic oxidative stress generated in the host cells due to decreased activity of selenoproteins, especially glutathione peroxidases (GPX), may lower the immune system and trigger genome mutations in the parasitic virus that generally change the virus from being benign or mildly pathogenic to become highly virulent (Beck et al. 2000; Nelson et al. 2001). Such RNA viral mutations are faster and long-lived in Se-deficient than in healthy individuals (Harthill 2011).
Se-deficient infected hosts get benefits from Se supplementation, which result in decrease of virus mutation frequency and load, and enhanced immune competence with better outcomes for viral infections (Beck et al. 2003; Harthill 2011). This has been corroborated for at least influenza virus type A and Coxsackievirus B3 (CVB3), human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) (Beck et al. 1995; Broome et al. 2004; Kupka et al. 2004; Nelson et al. 2001). Nonetheless, mutated virulent RNA viruses persist as pathogenic and infectious parasites in the hosts and can be transferred to individuals with Se-sufficient status (Harthill 2011).
Very recently, an epidemic caused by a novel coronavirus (COVID-19 or 2019‐CoV) belonging to the same group of β-coronaviruses as severe acute respiratory syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) (Cohen and Normile 2020), is spreading worldwide while threating human health and threatening the world economy. So far, no pharmacological treatment or vaccine exist. In any case, pharmaceutical drugs are usually ineffective against RNA viruses (Lyons 2019), while vaccines against coronaviruses carry an unacceptable risk of paradoxical immune enhancement (Tirado and Yoon 2003; Bolles et al. 2011; Ricke and Malone 2020) Therefore, alternative or supplementary natural treatments should be considered to reduce the viral load in the hosts and enhance their immune system. Similar to Zn, Se supplementation to COVID‐19 infected people with low Se blood levels could be an option to explore as a natural treatment of this virus (Zhang and Liu 2020). In this context, Se biofortification programs attain incremental importance, as Se enriched food crop is higher in nutritional components compared to Se intake via tablets, and thus provides more benefits to infected consumers. If the food is additionally enriched with Zn and Fe besides Se, its potential in contrasting viral infection could be significantly increased.
Conclusions and future prospects
The element Se may serve in different agrotechnological applications. Among them, agronomic biofortification through Se fertilizers and conventional breeding are likely the most widespread and accepted methods to combat the Se deficiency worldwide. Circular systems where Se phytoremediation is combined with biofortification strategies and genetic engineering of crops, although promising in the context of sustainable agriculture, are still poorly developed because of some practical limitations. In contrast, nanosized Se biofortification is increasingly gaining attention from scientists. Further investigation is needed to understand if the use of SeNPs is safe to consumers, and which chemical modifications they might undergo in the environment and during food processing. Studies on crop biofortification with multiple micronutrients and the use of PGPR in agronomic biofortification are only at the beginning, but might represent a future and promising area of Se biofortification research.
Selenium biofortification primarily aims to enrich crops with Se-species and antioxidant compounds with positive impact on human (and animal) nutrition and health. The role of Se-enriched food in preventing or contrasting viral infections in particular, is of great relevance in the recent scenario of a viral disease pandemic and represents a field of research that deserves a broad investigation. Se biofortification could also be used to increase crop yield under sub-optimal conditions, mitigating the negative effects of such environments on plant physiology, while increasing plant antioxidant properties and content in healthy phytochemicals. On this account, studies attempting optimization of Se biofortification strategies for improving food crop nutritional under challenging environments are of great interest on a global scale.
Change history
16 November 2020
A funding note has been added.
References
Alfthan G, Eurola M, Ekholm P, Venäläinen E-R, Root T, Korkalainen K, Hartikainen H, Salminen P, Hietaniemi V, Aspila P, Aro A, for the Selenium Working Group (2015) Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland. From deficiency to optimal selenium status of the population. J Trace Elem Med Biol 31:142–147. https://doi.org/10.1016/j.jtemb.2014.04.009
Agalou A, Roussis A, Spaink HP (2005) The Arabidopsis selenium-binding protein confers tolerance to toxic levels of selenium. Funct Plant Biol 32:881–890. https://doi.org/10.1071/FP05090
Ashraf MA, Akbar A, Parveen A, Rasheed R, Hussain I, Iqbal M (2018) Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol Biochem 123:268–280. https://doi.org/10.1016/j.plaphy.2017.12.023
Ates D, Sever T, Aldemir S, Yagmur B, Temel HY, Kaya HB, Alsaleh A, Kahraman A, Ozkan H, Vandenberg A, Tanyolac B (2016) Identification QTLs Controlling Genes for Se Uptake in Lentil Seeds. PLoS One 11(3):e0149210. https://doi.org/10.1371/journal.pone.0149210
Ávila FW, Yang Y, Faquin V, Ramos SJ, Guilherme LR, Thannhauser T, Li L (2014) Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem 165:578–586. https://doi.org/10.1016/j.foodchem.2014.05.134
Babajani A, Iranbakhsh A, Oraghi Ardebili Z, Eslami B (2019) Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ Sci Pollut Res Int 26(24):24430–24444. https://doi.org/10.1007/s11356-019-05676-z
Bachiega P, Salgado JM, de Carvalho JE, Ruiz A, Schwarz K, Tezotto T, Morzelle MC (2016) Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea, var. Italica) biofortified with selenium. Food Chem 190:771–776. https://doi.org/10.1016/j.foodchem.2015.06.024
Bajaj M, Eiche E, Neumann T, Winter J, Gallert C (2011) Hazardous concentrations of selenium in soil and groundwater in North-West India. J Hazard Mat 189:640–646. https://doi.org/10.1016/j.jhazmat.2011.01.086
Bañuelos GS, Robinson J, Da Roche J (2009) Developing selenium-enriched animal feed and biofuel from canola planted for managing Se-laden drainage waters in the Westside of Central California. Int J Phytorem 12:243–254. https://doi.org/10.1080/15226510903563850
Bañuelos GS, Arroyo I, Pickering IJ, Yang SI, Freeman JL (2015) Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem 166:603–608. https://doi.org/10.1016/j.foodchem.2014.06.071
Bañuelos GS, Lin Z-Q, Broadley MR (2017) Selenium biofortification. In: Pilon-Smits EAH, Winkel LHE, Lin Z-Q (eds) Selenium in plants: molecular, physiological, ecological and evolutionary aspects. Springer International Publishing, Cham, pp 231–256. https://doi.org/10.1007/978-3-319-56249-0
Bañuelos GS, Freeman J, Arroyo I (2019) Accumulation and speciation of selenium in biofortified vegetables grown under high boron and saline field conditions. Food Chem 5:100073. https://doi.org/10.1016/j.fochx.2019.100073
Bărbieru OG, Dimitriu L, Călin M, Raut I, Constantinescu Aruxandei D, Oancea F (2019) Plant biostimulants based on selenium nanoparticles biosynthesized by Trichoderma strains. Proceedings 29(1):95. https://doi.org/10.3390/proceedings2019029095
Barickman TC, Kopsell DA, Sams CE (2013) Selenium influences glucosinolate and isothiocyanates and increases sulfur uptake in Arabidopsis thaliana and rapid-cycling Brassica oleracea. J Agric Food Chem 61(1):202–209. https://doi.org/10.1021/jf3037227
Beck MA, Shi Q, Morris VC, Levander OA (1995) Rapid genomic evolution of a non-virulent Coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433–436. https://doi.org/10.1038/nm0595-433
Beck MA (2000) Nutritionally induced oxidative stress: effect on viral disease. Am J Clin Nutr 71(suppl):1676S–1679S. https://doi.org/10.1093/ajcn/71.6.1676S
Beck MA, Levander OA, Handy J (2003) Selenium deficiency and viral infection. J Nutr 133(5):1463S-1467S. https://doi.org/10.1093/jn/133.5.1463S
Bocchini M, D’Amato R, Ciancaleoni S, Fontanella MC, Palmerini CA, Beone GM, Onofri A, Negri V, Marconi G, Albertini E, Businelli D (2018) Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in Zea mays L. by inducing a higher drought tolerance. Front Plant Sci 9:389. https://doi.org/10.3389/fpls.2018.00389
Bohrer AS, Yoshimoto N, Sekiguchi A, Rykulski N, Saito K, Takahashi H (2015) Alternative translational initiation of ATP sulfurylase underlying dual localization of sulfate assimilation pathways in plastids and cytosol in Arabidopsis thaliana. Front Plant Sci 5:750. https://doi.org/10.3389/fpls.2014.00750
Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, Funkhouser W, Gralinski L, Totura A, Heise M, Baric RS (2011) A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol 85(23):12201–12215. https://doi.org/10.1128/JVI.06048-11
Boyd R (2011) Selenium stories. Nature Chem 3:570 (2011). https://doi.org/10.1038/nchem.1076
Broadley MR, White PJ, Bryson RJ, Meacham MC, Bowen HC, Johnson SE, Hawkesford MJ, McGrath SP, Zhao FJ, Breward N, Harriman M, Tucker M (2006) Biofortification of UK food crops with selenium. Proc Nutr Soc 65(2):169–181. https://doi.org/10.1079/pns2006490
Broadley MR, Alcock J, Alford J, Cartwright P, Foot I, Fairweather-Tait SJ, Hart DJ, Hurst R, Knott P, McGrath SP, Meacham MC (2010) Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 332:5–18. https://doi.org/10.1007/s11104-009-0234-4
Broome CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ (2004) An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 80:154–164. https://doi.org/10.1093/ajcn/80.1.154
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17. https://doi.org/10.1007/s11104-007-9466-3
Cakmak I, Marzorati M, Van den Abbeele P, Hora K, Tjalling Holwerda H, Yazici MA, Savasli E, Neri J, Du Laing G (2020) Fate and bioaccessibility of iodine in food prepared from agronomically biofortified wheat and rice and impact of cofertilization with zinc and selenium. J Agr Food Chem 68(6):1525–1535. https://doi.org/10.1021/acs.jafc.9b05912
Carvalho SMP, Vasconcelos MW (2013) Producing more with less: strategies and novel technologies for plant-based food biofortification. Food Res Int 54:961–971. https://doi.org/10.1016/j.foodres.2012.12.021
Chan Q, Afton SE, Caruso JA (2010) Selenium speciation profiles in selenite-enriched soybean (Glycine Max) by HPLC-ICPMS and ESI-ITMS. Metallomics 2(2):147–153. https://doi.org/10.1039/b916194e
Chauhan R, Awasthi S, Srivastava S, Dwivedi S, Pilon-Smits EAH, Dhankher OP, Rudra D (2019) Understanding selenium metabolism in plants and its role as a beneficial element. Crit Rev Environ Sci Tech 49:1937–1958. https://doi.org/10.1080/10643389.2019.1598240
Chen M, Zeng L, Luo X, Mehboob MZ, Ao T, Lang M (2019) Identification and functional characterization of a novel selenocysteine methyltransferase from Brassica juncea L. J Exp Bot 70(21):6401–6416. https://doi.org/10.1093/jxb/erz390
Chilimba AD, Young SD, Black CR, Meacham MC, Lammel J, Broadley MR (2012) Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crop Res 125:118–128. https://doi.org/10.1016/j.fcr.2011.08.014
Christophersen OA, Lyons G, Haug A, Steinnes E (2012) Sources of Heavy Metals and Metalloids in Soils. In: Alloway BJ (ed) Heavy metals in soils: trace elements and metalloids in soils and their bioavailability, 3rd edn. Environ Pollut. Springer, Dordrecht, pp 11–50. https://doi.org/10.1007/978-94-007-4470-7
Cohen J, Normile D (2020) New SARS-like virus in China triggers alarm. Science 367(6475):234–235. https://doi.org/10.1126/science.367.6475.234
Combs GF Jr (2001) Selenium in global food systems. Brit J Nutr 85(5):517–547. https://doi.org/10.1079/bjn2000280
Constantinescu-Aruxandei D, Frîncu RM, Capră L, Oancea F (2018) Selenium analysis and speciation in dietary supplements based on next-generation selenium ingredients. Nutrients 10:1466. https://doi.org/10.3390/nu10101466
Curtin DR, Hanson TN, Lindley RC (2006) Butler Selenium concentration in wheat (Triticum aestivum) grain as influenced by method, rate, and timing of sodium selenate application. New Zeal J Crop Hort Sci 34:329–339. https://doi.org/10.1080/01140671.2006.9514423
D’Amato R, Proietti P, Onofri A, Regni L, Esposto S, Servili M, Businelli D, Selvaggini R (2017) Biofortification (Se): Does it increase the content of phenolic compounds in virgin olive oil (VOO)? PLoS One 12(4):e0176580. https://doi.org/10.1371/journal.pone.0176580
D’Amato R, De Feudis M, Hasuoka PE, Regni L, Pacheco PH, Onofri A, Businelli D, Proietti P (2018a) The selenium supplementation influences olive tree production and oil stability against oxidation and can alleviate the water deficiency effects. Front Plant Sci 9:1191. https://doi.org/10.3389/fpls.2018.01191
D’Amato R, Fontanella MC, Falcinelli B, Beone GM, Bravi E, Marconi O, Benincasa P, Businelli D (2018b) Selenium biofortification in rice (Oryza sativa L.) sprouting: effects on Se yield and nutritional traits with focus on phenolic acid profile. J Agric Food Chem 66(16):4082–4090. https://doi.org/10.1021/acs.jafc.8b00127
D’Amato R, De Feudis M, Guiducci M, Businelli D (2019) Zea mays L. Grain: Increase in nutraceutical and antioxidant properties due to Se fortification in low and high water regimes. J Agric Food Chem 67(25):7050–7059. https://doi.org/10.1021/acs.jafc.9b02446
D’Amato R, Regni L, Falcinelli B, Mattioli S, Benincasa P, Dal Bosco A, Pacheco P, Proietti P, Troni E, Santi C, Businelli D (2020) Current knowledge on selenium biofortification to improve the nutraceutical profile of food: a comprehensive review. J Agr Food Chem 68:4075–4097. https://doi.org/10.1021/acs.jafc.0c00172
Dall’Acqua S, Ertani A, Pilon-Smits EAH, Fabrega-Prats M, Schiavon M (2019) Selenium Biofortification differentially affects sulfur metabolism and accumulation of phytochemicals in two rocket species (Eruca Sativa Mill. and Diplotaxis Tenuifolia) grown in hydroponics. Plants (Basel) 8(3):68. https://doi.org/10.3390/plants8030068
Davis CD, Selenium Supplementation and Cancer Prevention (2012) Curr Nutr Rep 1:16–23. https://doi.org/10.1007/s13668-011-0003-x
De Feudis M, D’Amato R, Businelli D, Guiducci M (2019) Fate of selenium in soil: A case study in a maize (Zea mays L.) field under two irrigation regimes and fertilized with sodium selenite. Sci Tot Environ 659:131–139. https://doi.org/10.1016/j.scitotenv.2018.12.200
Deng XF, Zhao ZQ, Han ZY, Huang LQ, Lv CH, Zhang ZH, Zhang HQ, Liu XW (2019) Selenium uptake and fruit quality of pear (Pyrus communis L.) treated with foliar Se application. J Plant Nutr Soil Sci 182:637–646. https://doi.org/10.1002/jpln.201800295
Dhillon SK, Dhillon KS (2009) Phytoremediation of selenium-contaminated soils: the efficiency of different cropping systems. Soil Use Manag 25:441–453. https://doi.org/10.1111/j.1475-2743.2009.00217.x
Diao M, Ma L, Wang J, Cui J, Fu A, Liu H (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33:671–682. https://doi.org/10.1007/s00344-014-9416-2
Dinh QT, Cui Z, Huang J, Tran TAT, Wang D, Yang W, Zhou F, Wang M, Yu D, Liang D (2018) Selenium distribution in the Chinese environment and its relationship with human health: a review. Environ Int 112:294–309. https://doi.org/10.1016/j.envint.2017.12.035
Domokos-Szabolcsy E, Marton L, Sztrik A, Babka B, Prokisch J, Fari M (2012) Accumulation of red elemental selenium nanoparticles and their biological effects in Nicotiana tabacum. Plant Growth Regul 68:525–531. https://doi.org/10.1007/s10725-012-9735-x
Dos Reis AR, El-Ramady H, Santos EF, Gratão PL, Schomburg L (2017) Overview of selenium deficiency and toxicity worldwide: Affected areas, selenium-related health issues, and case studies. In: Pilon-Smits EAH, Winkel LHE, Lin Z-Q (eds) Selenium in plants: molecular, physiological, ecological and evolutionary aspects. Springer International Publishing, Cham, pp 209–230. https://doi.org/10.1007/978-3-319-56249-0
Duncan EG, Maher WA, Jagtap R, Krikowa F, Roper MM, O’Sullivan CA (2017) Selenium speciation in wheat grain varies in the presence of nitrogen and sulphur fertilisers. Environ Geochem Health 39:955–966. https://doi.org/10.1007/s10653-016-9857-6
Durán P, Acuña JJ, Jorquera MA, Azcón R, Borie F, Cornejo P, Mora ML (2013) Enhanced selenium content in wheat grain by co-inoculation of selenobacteria and arbuscular mycorrhizal fungi: A preliminary study as a potential Se biofortification strategy. J Cereal Sci 57(3):275–280. https://doi.org/10.1016/j.jcs.2012.11.012
Durán P, Acuña JJ, Jorquera MA, Azcón R, Paredes C, Rengel Z, Mora ML (2014) En- dophytic bacteria from selenium-supplemented wheat plants could be useful for plant-growth promotion, biofortification and Gaeumanno- mycesgraminis biocontrol in wheat production. Biol Fert Soils 50:983–990
Eich-Greatorex S, Sogn TA, Øgaard AF, Aasen I (2007) Plant availability of inorganic and organic selenium fertiliser as influenced by soil organic matter content and pH. Nutr Cycl Agroecosyst 79:221–231. https://doi.org/10.1007/s10705-007-9109-3
El Mehdawi AF, Jiang Y, Guignardi ZS, Esmat A, Pilon M, Pilon-Smits EAH, Schiavon M (2018) Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol 217:194–205. https://doi.org/10.1111/nph.14838
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA (2019) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem 137:144–153. https://doi.org/10.1016/j.plaphy.2019.02.004
Emmert SW, Desai D, Amin S, Richie JP Jr (2010) Enhanced Nrf2-dependent induction of glutathione in mouse embryonic fibroblasts by isoselenocyanate analog of sulforaphane. Bioorg Med Chem Lett 20(8):2675–2679. https://doi.org/10.1016/j.bmcl.2010.01.044
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147. https://doi.org/10.3389/fpls.2017.01147
Freeman JL, Lindblom SD, Quinn CF, Fakra S, Marcus MA, Pilon-Smits EA (2007) Selenium accumulation protects plants from herbivory by Orthoptera via toxicity and deterrence. New Phytol 75:490–500. https://doi.org/10.1111/j.1469-8137.2007.02119.x
Freeman JL, Quinn CF, Lindblom SD, Klamper EM, Pilon-Smits EA (2009) Selenium protects the hyperaccumulator Stanleya pinnata against black-tailed prairie dog herbivory in native seleniferous habitats. Am J Bot 96(6):1075–1085. https://doi.org/10.3732/ajb.0800287
Fordyce FM (2013) Selenium deficiency and toxicity in the environment. In: Selinus O (ed) Essentials of Medical Geology. Springer, Dordrecht, pp 375–416
Freschi L (2013) Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci 4:398. https://doi.org/10.3389/fpls.2013.00398
Garg M, Sharma N, Sharma S, Kapoor P, Kumar A, Chunduri V, Arora P (2018) Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front Nutr 5:12. https://doi.org/10.3389/fnut.2018.00012
Golob A, Novak T, Maršić NK, Šircelj H, Stibilj V, Jerše A, Kroflič A, Germ M (2020) Biofortification with selenium and iodine changes morphological properties of Brassica oleracea L. var. gongylodes) and increases their contents in tubers. Plant Physiol Biochem 150:234–243. https://doi.org/10.1016/j.plaphy.2020.02.044
Golubkina N, Zamana S, Seredin T, Poluboyarinov P, Sokolov S, Baranova H, Krivenkov L, Pietrantonio L, Caruso G (2019) Effect of selenium biofortification and beneficial microorganism inoculation on yield, quality and antioxidant properties of shallot bulbs. Plants 8(4):102. https://doi.org/10.3390/plants8040102
Golubkina N, Kekina H, Caruso G (2018) Yield, quality and antioxidant properties of Indian Mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants (Basel). 7(4). https://doi.org/10.3390/plants7040080
Ghosh S, Biswas AK (2017) Selenium modulates growth and thiol metabolism in wheat (Triticum aestivum L.) during arsenic stress. Am J Plant Sci 8:363. https://doi.org/10.4236/ajps.2017.83026
Guardado-Félix D, Antunes-Ricardo M, Rocha-Pizaña M, Martínez-Torres AC, Gutiérrez-Uribe J, Serna Saldivar S (2019) Chickpea (Cicer arietinum L.) sprouts containing supranutritional levels of selenium decrease tumor growth of colon cancer cells xenografted in immune-suppressed mice. J Func Foods 53:76–84. https://doi.org/10.1016/j.jff.2018.07.003
Guillin OM, Vindry C, Ohlmann T, Chavatte L (2019) Selenium, selenoproteins and viral Infection. Nutrients 11(9). https://doi.org/10.3390/nu11092101
Gupta UC, Gupta SC (2000) Selenium in soils and crops, its deficiencies in livestock and humans: implications for management. Commun Soil Sci Plan 31:1791–1807. https://doi.org/10.1080/00103620009370538
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074. https://doi.org/10.3389/fpls.2016.02074
Habibi G (2017) Physiological, photochemical and ionic responses of sunflower seedlings to exogenous selenium supply under salt stress. Acta Physiol Plant 39:213. https://doi.org/10.1007/s11738-017-2517-3
Hajiboland R, Amjad L (2008) The effects of selenate and sulphate supply on the accumulation and volatilization of Se by cabbage, kohlrabi and alfalfa plants grown hydroponically. Agric Food Sci 17(2):177–189. https://doi.org/10.2137/145960608785328242
Hajiboland R, Rahmat S, Zeinalzadeh N, Farsad-Akhtar N, Hosseinpour-Feizi MA (2019) Senescence is delayed by selenium in oilseed rape plants. J Trace Elements Med Biol 55:96–106. https://doi.org/10.1016/j.jtemb.2019.06.005
Harthill M (2011) Review: micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol Trace Elem Res 143(3):1325–1336. https://doi.org/10.1007/s12011-011-8977-1
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18:309–318. https://doi.org/10.1016/j.jtemb.2005.02.009
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143:1758–1776. https://doi.org/10.1007/s12011-011-8998-9
Hasanuzzaman M, Hossain MA, Fujita M (2011) Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res 143(3):1704–1721. https://doi.org/10.1007/s12011-011-8958-4
Haug A, Graham R, Christophersen O, Lyons G (2007) How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb Ecol Health Dis 19(4):209–228. https://doi.org/10.1080/08910600701698986
Hawrylak-Nowak B (2008) Enhanced selenium content in sweet basil (Ocimum Basilicum L.) by foliar fertilization. Veg Crops Res Bull 69(1):63–72. https://doi.org/10.2478/v10032-008-0021-4
Hawrylak-Nowak B (2009) Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol Trace Elem Res 132:259–269. https://doi.org/10.1007/s12011-009-8402-1
Hawrylak-Nowak B (2013) Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul 70:149–157. https://doi.org/10.1007/s10725-013-9788-5
Hawrylak-Nowak B, Dresler S, Rubinowska K, Matraszek-Gawron R, Woch W, Hasanuzzaman M (2018) Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol Biochem 127:446–456. https://doi.org/10.1016/j.plaphy.2018.04.018
He Y, Xiang Y, Zhou Y, Yang Y, Zhang J, Huang H, Shang C, Luo L, Gao J, Tang L (2018) Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ Res 164:288–301. https://doi.org/10.1016/j.envres.2018.02.037
Huang G, Ding C, Yu X, Yang Z, Zhang T, Wang X (2018) Characteristics of time-dependent selenium biofortification of rice (Oryza sativa L.). J Agric Food Chem 66(47):12490–12497. https://doi.org/10.1021/acs.jafc.8b04502
Hung CY, Holliday BM, Kaur H, Yadav R, Kittur FS, Xie J (2012) Identification and characterization of selenate- and selenite-responsive genes in a Se-hyperaccumulator Astragalus racemosus. Mol Biol Rep 39(7):7635–7646. https://doi.org/10.1007/s11033-012-1598-8
Hunter WJ, Manter DK (2008) Bio-reduction of selenite to elemental red selenium by Tetrathiobacter kashmirensis. Curr Microbiol 57:83–88. https://doi.org/10.1007/s00284-008-9160-6
Hunter WJ (2014) Rhizobium seleniti reducens protein showing selenite reductase activity. Curr Microbiol 68:311–316. https://doi.org/10.1007/s00284-013-0474-7
Husen A, Siddiqi KS (2014) Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnology 12:28. https://doi.org/10.1186/s12951-014-0028-6
Hussein HA, Darwesh OM, Mekki BB, El-Hallouty SM (2019) Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotech Rep 24:e00377. https://doi.org/10.1016/j.btre.2019.e00377
Institute of Medicine (2000) Dietary reference intakes for vitamin c, vitamin e, selenium, and carotenoids, pp 284–324. The National Academies Press, Washington
Iqbal M, Hussain I, Liaqat H, Ashraf MA, Rasheed R, Rehman AU (2015) Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol Biochem 94:95–103. https://doi.org/10.1016/j.plaphy.2015.05.012
Islam MZ, Park BJ, Kang HM, Lee YT (2020) Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem 309:125763. https://doi.org/10.1016/j.foodchem.2019.125763
Jha AB, Warkentin TD (2020) Biofortification of pulse crops: status and future perspectives. Plants (Basel). 6;9(1). https://doi.org/10.3390/plants9010073
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na + accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039. https://doi.org/10.1038/srep42039
Jiang L, Chen Z, Gao Q, Ci L, Cao S, Han Y, Wang W (2016) Loss-of-function mutations in the APX1 gene result in enhanced selenium tolerance in Arabidopsis thaliana. Plant Cell Environ 39(10):2133–2144. https://doi.org/10.1111/pce.12762
Jiang L, Yang J, Liu C, Chen Z, Yao Z, Cao S (2020) Overexpression of ethylene response factor ERF96 gene enhances selenium tolerance in Arabidopsis. Plant Physiol Biochem 149:294–300. https://doi.org/10.1016/j.plaphy.2020.02.024
Jiang Y, Schiavon M, Lima LW, Tripti, Jones RR, El Mehdawi AF, Royer S, Zeng Z, Hu Y, Pilon-Smits EAH, Pilon M (2018) Comparison of ATP sulfurylase 2 from selenium hyperaccumulator Stanleya pinnata and non-accumulator Stanleya elata reveals differential intracellular localization and enzyme activity levels. Biochim Biophys Acta Gen Subj S0304-4165(18)30076-X. https://doi.org/10.1016/j.bbagen.2018.03.014
Jones GD, Droz B, Greve P, Gottschalk P, Poffet D, McGrath SP, Seneviratne SI, Smith P, Winkel LH (2017) Selenium deficiency risk predicted to increase under future climate change. Proc Natl Acad Sci USA 114(11):2848–2853. https://doi.org/10.1073/pnas.1611576114
Jóźwiak W, Politycka B. (2019) Effect of selenium on alleviating oxidative stress caused by a water deficit in Cucumber Roots. Plants (Basel). 11;8(7). https://doi.org/10.3390/plants8070217
Joy EJM, Kalimbira AA, Gashu D, Ferguson EL, Sturgess J, Dangour AD, Banda L, Chiutsi-Phiri G, Bailey EH et al (2019) Can selenium deficiency in Malawi be alleviated through consumption of agro-biofortified maize flour? Study protocol for a randomised, double-blind, controlled trial. Trials. 20(1):795. https://doi.org/10.1186/s13063-019-3894-2
Juárez-Maldonado A, Ortega-Ortíz H, Morales-Díaz AB, González-Morales S, Morelos-Moreno Á, Cabrera-De la Fuente M, Sandoval-Rangel A, Cadenas-Pliego G, Benavides-Mendoza A (2019) Nanoparticles and nanomaterials as plant biostimulants. Int J Mol Sci 20(1):162. https://doi.org/10.3390/ijms20010162
Kamran M, Parveen A, Ahmar S, Malik Z, Hussain S, Chattha MS, Saleem MH, Adil M, Heidari P, Chen JT (2019) An Overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int J Mol Sci Dec 24;21(1). https://doi.org/10.3390/ijms21010148
Kieliszek M (2019) Selenium-fascinating microelement, properties and sources in food. Molecules. 24(7). https://doi.org/10.3390/molecules24071298
Kikkert J, Hale B, Berkelaar E (2013) Selenium accumulation in durum wheat and spring canola as a function of amending soils with selenite, selenate and or sulphate. Plant Soil 372:629–641. https://doi.org/10.1007/s11104-013-1773-2
Kim SY, Park JE, Kim EO, Lim SJ, Nam EJ, Yun JH, Yoo G, Oh SR, Kim HS, Nho CW (2018) Exposure of kale root to NaCl and Na2SeO3 increases isothiocyanate levels and Nrf2 signalling without reducing plant root growth. Sci Rep 8(1):3999. https://doi.org/10.1038/s41598-018-22411-9
Kolbert Z, Molnï RÏRD, Szőllősi RK, Feigl GB, Erdei LS, Ï Rdï GA (2018) Nitro-oxidative stress correlates with Se tolerance of Astragalus species. Plant Cell Physiol 59(9):1827–1843. https://doi.org/10.1093/pcp/pcy099
Kornaś A, Filek M, Sieprawska A, Bednarska-Kozakiewicz E, Gawrońska K, Miszalski Z (2019) Foliar application of selenium for protection against the first stages of mycotoxin infection of crop plant leaves. J Sci Food Agric 99(1):482–485. https://doi.org/10.1002/jsfa.9145
Kupka R, Msamanga GI, Spiegelman D, Morris S, Mugusi F, Hunter DJ, Fawzi WW (2004) Selenium status is associated with accelerated HIV disease progression among HIV-1-infected pregnant women in Tanzania. J Nutr 134(10):2556–2560. https://doi.org/10.1093/jn/134.10.2556
Li Y, Zhu N, Liang X, Zheng L, Zhang C, Li YF, Zhang Z, Gao Y, Zhao J (2020) A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotoxicol Environ Saf 189:109955. https://doi.org/10.1016/j.ecoenv.2019.109955
Lima LW, Pilon-Smits EAH, Schiavon M (2018) Mechanisms of selenium hyperaccumulation in plants: A survey of molecular, biochemical and ecological cues. Biochim Biophys Acta Gen Subj S0304-4165(18)30090-4. https://doi.org/10.1016/j.bbagen.2018.03.028
Lindblom SD, Fakra SC, Landon J, Schulz P, Tracy B, Pilon-Smits EAH (2014) Inoculation of selenium hyperaccumulator Stanleya pinnata and related non-accumulator Stanleya elata with hyperaccumulator rhizosphere fungi—investigation of effects on Se accumulation and speciation. Physiol Plant 150:107–118. https://doi.org/10.1111/ppl.12094
Liu K, Cai M, Hu C, Sun X, Cheng Q, Jia W, Yang T, Nie M, Zhao X (2019) Selenium (Se) reduces Sclerotinia stem rot disease incidence of oilseed rape by increasing plant Se concentration and shifting soil microbial community and functional profiles. Environ Pollut 254(Pt B):113051. https://doi.org/10.1016/j.envpol.2019.113051
Lyons G (2010) Selenium in cereals: Improving the efficiency of agronomic biofortification in the UK. Plant Soil 332:1–4. https://doi.org/10.1007/s11104-010-0282-9
Lyons G (2019) Selenium-biofortified herbs as antivirals? In Perspectives, Technologies and Advancements: proceedings of the 6th International Conference on Selenium in the Environment and Human Health. Yangling, Shaanxi, China
Mangiapane E, Pessione A, Pessione E (2014) Selenium and selenoproteins: an overview on different biological systems. Curr Protein Pept Sci 15(6):598–607. https://doi.org/10.2174/1389203715666140608151134
Malerba M, Cerana R (2018) Effect of selenium on the responses induced by heat stress in plant cell cultures. Plants (Basel) 7(3). https://doi.org/10.3390/plants7030064
Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H (2012) Selenium antagonizes the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot 77:242–248. https://doi.org/10.1016/j.envexpbot.2011.12.001
Mall RK, Gupta A, Sonkar G (2017) Effect of climate change on agricultural crops. Crop modification, nutrition, and food production. Curr Dev Biotech Bioeng 23–46. https://doi.org/10.1016/B978-0-444-63661-4.00002-5
Mao H, Wang J, Wang Z, Zan Y, Lyons G, Zou C (2014) Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J Soil Sci Plant Nutr 14:459–470. https://doi.org/10.4067/S0718-95162014005000036
Matich AJ, McKenzie MJ, Lill RE, Brummell DA, McGhie TK, Chen RK, Rowan DD (2012) Selenoglucosinolates and their metabolites produced in Brassica spp. fertilised with sodium selenate. Phytochemistry 75:140–152. https://doi.org/10.1016/j.phytochem.2011.11.021
Matich AJ, McKenzie MJ, Lill RE, McGhie TK, Chen RK, Rowan DD (2015) Distribution of selenoglucosinolates and their metabolites in Brassica treated with sodium selenate. J Agric Food Chem 63(7):1896–1905. https://doi.org/10.1021/jf505963c
McKenzie MJ, Chen RKY, Leung S, Joshi S, Rippon PE, Joyce NI, McManus MT (2017) Selenium treatment differentially affects sulfur metabolism in high and low glucosinolate producing cultivars of broccoli (Brassica oleracea L.). Plant Physiol Biochem 121:176–186. https://doi.org/10.1016/j.plaphy.2017.10.027
McKenzie M, Matich A, Hunter D, Esfandiari A, Trolove S, Chen R, Lill R (2019) Selenium application during radish (Raphanus sativus) plant development alters glucosinolate metabolic gene expression and results in the production of 4-(methylseleno)but-3-enyl glucosinolate. Plants (Basel) 8(10). https://doi.org/10.3390/plants8100427
Mechora Š (2019) Selenium as a protective agent against pests: a review. Plants (Basel). 8(8). https://doi.org/10.3390/plants8080262
Mehdi Y, Hornick JL, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18(3):3292–3311. https://doi.org/10.3390/molecules18033292
Melrose J (2019) The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 7(3):62. https://doi.org/10.3390/biomedicines7030062
Miller DD, Welch RM (2013) Food system strategies for preventing micronutrient malnutrition. Food Policy 42:115–128. https://doi.org/10.1016/j.foodpol.2013.06.008
Mimmo T, Tiziani R, Valentinuzzi F, Lucini L, Nicoletto C, Sambo P, Scampicchio M, Pii Y, Cesco S (2017) Selenium Biofortification in Fragaria × ananassa: Implications on Strawberry Fruits Quality, Content of Bioactive Health Beneficial Compounds and Metabolomic Profile. Front Plant Sci 8:1887. https://doi.org/10.3389/fpls.2017.01887
Mora ML, Durán P, Acuña J, Cartes P, Demanet R, Gianfreda L (2015) Improving selenium status in plant nutrition and quality. J Soil Sci Plant Nutr 15(2):486–503. https://doi.org/10.4067/S0718-95162015005000041
Morales-Espinoza MC, Cadenas-Pliego G, Pérez-Alvarez M, Hernández-Fuentes AD, Cabrera de la Fuente M, Benavides-Mendoza A, Valdés-Reyna J, Juárez-Maldonado A (2019) Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl Stress. Molecules 24(17). https://doi.org/10.3390/molecules24173030
Natasha, Shahid M, Niazi NK, Khalid S, Murtaza B, Bibi I, Rashid MI (2018) A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Pollut 234:915–934. https://doi.org/10.1016/j.envpol.2017.12.019
Nawaz F, Ashraf MY, Ahmad R, Waraich EA, Shabbir RN, Bukhari MA (2015) Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem 175:350–357. https://doi.org/10.1016/j.foodchem.2014.11.147
Nawaz F, Naeem M, Ashraf MY, Tahir MN, Zulfiqar B, Salahuddin M, Shabbir RN, Aslam M (2016) Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front Plant Sci 27:1438. https://doi.org/10.3389/fpls.2016.01438
Nayantara, Kaur P (2018) Biosynthesis of nanoparticles using eco-friendly factories and their role in plant pathogenicity: a review. Biotech Res Innov 2(1):63–73. https://doi.org/10.1016/j.biori.2018.09.003
Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA, Beck MA (2001) Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J 15(10):1846–1848. https://doi.org/10.1096/fj.01-0115fje
Newman R, Waterland N, Moon Y, Tou JC (2019) Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention - a Review. Plant Foods Hum Nutr 74(4):449–460. https://doi.org/10.1007/s11130-019-00769-z
Ni TW, Staicu LC, Nemeth RS, Schwartz CL, Crawford D, Seligman JD, Hunter WJ, Pilon-Smits EA, Ackerson CJ (2015) Progress toward clonable inorganic nanoparticles. Nanoscale 7(41):17320–17327. https://doi.org/10.1039/c5nr04097c
Norton GJ, Deacon CM, Xiong L, Huang S, Meharg AA, Price AH (2010) Genetic mapping of the rice ionome in leaves and grain: identification of QTLs for 17 elements including arsenic, cadmium, iron and selenium. Plant Soil 329:139–153. https://doi.org/10.1007/s11104-009-0141-8
Ohlendorf HM, Santolo GM, Byron ER, Eisert MA (2020) Kesterson Reservoir: 30 years of selenium risk assessment and management. Integr Environ Assess Manag 16(2):257–268. https://doi.org/10.1002/ieam.4222
Oldfield JE (2002) Selenium world atlas: Updated Edition; Selenium-Tellurium Dev. Grimbergen, Assoc., Belgium. https://www.stda.org
Pannico A, El-Nakhel C, Graziani G, Kyriacou MC, Giordano M, Soteriou GA, Zarrelli A, Ritieni A, Pascale S, Rouphael Y (2020) Selenium biofortification impacts the nutritive value, polyphenolic content, and bioactive constitution of variable microgreens genotypes. Antioxidants (Basel) 9(4). https://doi.org/10.3390/antiox9040272
Pezzarossa B, Remorini D, Gentile ML, Massai R (2012) Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J Sci Food Agric 92(4):781–786. https://doi.org/10.1002/jsfa.4644
Pilon-Smits EA, LeDuc DL (2009) Phytoremediation of selenium using transgenic plants. Curr Opin Biotechnol 20(2):207–212. https://doi.org/10.1016/j.copbio.2009.02.001
Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M (2009) Physiological functions of beneficial elements. Curr Opin Plant Biol 12(3):267–274. https://doi.org/10.1016/j.pbi.2009.04.009
Pilon-Smits EAH (2019) On the ecology of selenium accumulation in plants. Plants (Basel) 30;8(7). https://doi.org/10.3390/plants8070197
Poblaciones MJ, Rodrigo S, Santamaria O, Chen Y, McGrath SP (2014) Selenium accumulation and speciation in biofortified chickpea (Cicer arietinum L.) under Mediterranean conditions. J Sci Food Agric 94(6):1101–1106. https://doi.org/10.1002/jsfa.6372
Premarathna L, McLaughlin MJ, Kirby JK, Hettiarachchi GM, Stacey S, Chittleborough DJ (2012) Selenate-enriched urea granules are a highly effective fertilizer for selenium biofortification of paddy rice grain. J Agric Food Chem 60(23):6037–6044. https://doi.org/10.1021/jf3005788
Prieto MA, López CJ, Simal-Gandara J (2019) Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv Food Nutr Res 90:305–350. https://doi.org/10.1016/bs.afnr.2019.02.008
Puccinelli M, Malorgio F, Rosellini I, Pezzarossa B (2019) Production of selenium-biofortified microgreens from selenium-enriched seeds of basil. J Sci Food Agric 99(12):5601–5605. https://doi.org/10.1002/jsfa.9826
Quinn CF, Freeman JL, Reynolds RJ, Cappa JJ, Fakra SC, Marcus MA, Lindblom SD, Quinn EK, Bennett LE, Pilon-Smits EA (2010) Selenium hyperaccumulation offers protection from cell disruptor herbivores. BMC Ecol 10:19. https://doi.org/10.1186/1472-6785-10-19
Rahmanto AS, Davies MJ (2012) Selenium-containing amino acids as direct and indirect antioxidants. IUBMB Life 64(11):863–871. https://doi.org/10.1002/iub.1084
Ramkissoon C, Degryse F, da Silva RC, Baird R, Young SD, Bailey EH, McLaughlin MJ (2019) Improving the efficacy of selenium fertilizers for wheat biofortification. Sci Rep 9(1):19520. https://doi.org/10.1038/s41598-019-55914-0
Ramos SJ, Yuan Y, Faquin V, Guilherme LR, Li L (2011) Evaluation of genotypic variation of broccoli (Brassica oleracea var. italic) in response to selenium treatment. J Agric Food Chem 59(8):3657–3665. https://doi.org/10.1021/jf104731f
Rashid MM, Lin Z-Q, Kaniz F (2016) Partitioning of SeNPs in the water soluble and the exchangeable fractions and effects of soil organic matter and incubation time. In: Global Advances in Selenium Research from Theory to Application. Eds. Bañuelos GS, Lin Z-Q, Moraes MF, Guilherme LRG, Dos Reis AR. Taylor & Francis Group, London, ISBN 978-1-138-02731-2
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Rayman MP (2020) Selenium intake, status, and health: a complex relationship. Hormones (Athens) 19(1):9–14. https://doi.org/10.1007/s42000-019-00125-5
Reis HPG, de Queiroz Barcelos JP, Silva VM, Santos EF, Tavanti RFR, Putti FF, Young SD, Broadley MR, White PJ, Dos Reis AR (2020) Agronomic biofortification with selenium impacts storage proteins in grains of upland rice. J Sci Food Agric 100(5):1990–1997. https://doi.org/10.1002/jsfa.10212
Ricke D, Malone R (2020) Medical countermeasures analysis of 2019-nCoV and vaccine risks for antibody-dependent enhancement (ADE). Preprints, 2020030138. https://doi.org/10.20944/preprints202003.0138.v1
Ríos JJ, Blasco B, Rosales MA, Sanchez-Rodriguez E, Leyva R, Cervilla LM, Romero L, Ruiz JM (2010) Response of nitrogen metabolism in lettuce plants subjected to different doses and forms of selenium. J Sci Food Agric 90(11):1914–1919. https://doi.org/10.1002/jsfa.4032
Robbins RJ, Keck AS, Banuelos G, Finley JW (2005) Cultivation conditions and selenium fertilization alter the phenolic profile, glucosinolate, and sulforaphane content of broccoli. J Med Food 8(2):204–214. https://doi.org/10.1089/jmf.2005.8.204
Ros G, van Rotterdam A, Bussink D, Bindraban P (2016) Selenium fertilization strategies for bio-fortification of food: an agro-ecosystem approach. Plant Soil 404:99–112. https://doi.org/10.1007/s11104-016-2830-4
Sabbagh M, Van Hoewyk D (2012) Malformed selenoproteins are removed by the ubiquitin–proteasome pathway in Stanleya pinnata. Plant Cell Physiol 53(3):555–564. https://doi.org/10.1093/pcp/pcs015
Saha U, Fayiga A, Sonon L (2017) Selenium in the soil-plant environment: A Review. Int J Appl Agr Sci 3(1):1–18. https://doi.org/10.11648/j.ijaas.20170301.11
Sajedi NA, Ardakani MR, Madani H, Naderi A, Miransari M (2011) The effects of selenium and other micronutrients on the antioxidant activities and yield of corn (Zea mays L.) under drought stress. Physiol Mol Biol Plants 17(3):215–222. https://doi.org/10.1007/s12298-011-0067-5
Sattar A, Cheema MA, Abbas T, Sher A, Ijaz M, Hussain M (2017) Separate and combined effects of silicon and selenium on salt tolerance of wheat plants. Russ J Plant Physiol 64:341–348. https://doi.org/10.1134/S1021443717030141
Schiavon M, Dall’Acqua S, Mietto A, Pilon-Smits EA, Sambo P, Masi A, Malagoli M (2013) Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J Agric Food Chem 61(44):10542–10554. https://doi.org/10.1021/jf4031822
Schiavon M, Berto C, Malagoli M, Trentin A, Sambo P, Dall’Acqua S, Pilon-Smits EA (2016) Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics, and amino acids. Front Plant Sci 7:1371. https://doi.org/10.3389/fpls.2016.01371
Schiavon M, Pilon-Smits EA (2017a) The fascinating facets of plant selenium accumulation - biochemistry, physiology, evolution and ecology. New Phytol 213(4):1582–1596. https://doi.org/10.1111/nph.14378
Schiavon M, Pilon-Smits EAH (2017b) Selenium Biofortification and Phytoremediation Phytotechnologies: A Review. J Environ Qual 46:10–19. https://doi.org/10.2134/jeq2016.09.0342
Sepúlveda I, Barrientos H, Mahn A, Moenne A (2013) Changes in SeMSC, glucosinolates and sulforaphane levels, and in proteome profile in broccoli (Brassica oleracea var. Italica) fertilized with sodium selenate. Molecules 18(5):5221–5234. https://doi.org/10.3390/molecules18055221
Shahid M, Niazi NK, Khalid S, Murtaza B, Bibi I, Rashid MI (2018) A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Poll 234:915–934. https://doi.org/10.1016/j.envpol.2017.12.019
Šindelářová K, Száková J, Tremlová J, Mestek O, Praus L, Kaňa A, Najmanová J, Tlustoš P (2015) The response of broccoli (Brassica oleracea convar. italica) varieties on foliar application of selenium: uptake, translocation, and speciation. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32(12):2027–2038. https://doi.org/10.1080/19440049.2015.1099744
Skrypnik L, Novikova A, Tokupova E (2019) Improvement of phenolic compounds, essential oil content and antioxidant properties of sweet basil (Ocimum basilicum L.) depending on type and concentration of selenium application. Plants (Basel) 8(11). https://doi.org/10.3390/plants8110458
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86(3):373–389. https://doi.org/10.1007/s11120-005-5222-9
Spallholz JE (2019) Selenomethionine and Methioninase: Selenium Free Radical Anticancer Activity. Methods Mol Biol 1866:199–210. https://doi.org/10.1007/978-1-4939-8796-2_15
Stibilj V, Smrkolj P, Jaćimović R, Osvald J (2011) Selenium uptake and distribution in chicory (Cichorium intybus L.) grown in an aeroponic system. Acta Agric Slov 97(3):189–196. https://doi.org/10.2478/v10014-011-0013-9
Stonehouse GC, McCarron BJ, Guignardi ZS, El Mehdawi AF, Lima LW, Fakra SC, Pilon-Smits EAH (2020) Selenium Metabolism in Hemp (Cannabis sativa L.)-Potential for Phytoremediation and Biofortification. Environ Sci Technol 54(7):4221–4230. https://doi.org/10.1021/acs.est.9b07747
Stroud JL, Broadley MR, Foot I, Fairweather-Tait SJ, Hart DJ, Hurst R, Knott P, Mowat H, Norman K, Scott P, Tucker M, White PJ, McGrath SP, Zhao FJ (2010) Soil factors affecting selenium concentration in wheat grain and the fate and speciation of Se fertilisers applied to soil. Plant Soil 332:19–30. https://doi.org/10.1007/s11104-009-0229-1
Subramanyam K, Du Laing G, Van Damme EJM (2019) Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front Plant Sci 10:116. https://doi.org/10.3389/fpls.2019.00116
Tan JA, Zhu W, Wang W, Li R, Hou S, Wang D et al (2002) Selenium in soil and endemic diseases in China. Sci Total Environ 284:227–235. https://doi.org/10.1016/s0048-9697(01)00889-0
Tan HW, Mo HY, Lau ATY, Xu YM (2018) Selenium species: current status and potentials in cancer prevention and therapy. Int J Mol Sci 20(1). https://doi.org/10.3390/ijms20010075
Tarrado-Castellarnau M, Cortés R, Zanuy M, Tarragó-Celada J, Polat IH, Hill R, Fan TW, Link W, Cascante M (2015) Methylseleninic acid promotes antitumour effects via nuclear FOXO3a translocation through Akt inhibition. Pharmacol Res 102:218–234. https://doi.org/10.1016/j.phrs.2015.09.009
Tian M, Xu X, Liu Y, Xie L, Pan S (2016) Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem 190:374–380. https://doi.org/10.1016/j.foodchem.2015.05.098
Tian M, Yang Y, Ávila FW, Fish T, Yuan H, Hui M, Pan S, Thannhauser TW, Li L (2018) Effects of Selenium Supplementation on Glucosinolate Biosynthesis in Broccoli. J Agric Food Chem 66(30):8036–8044. https://doi.org/10.1021/acs.jafc.8b03396
Tirado S, Yoon K (2003) Antibody-dependent enhancement of virus infection and disease. Vir Immunol 16:69–86. https://doi.org/10.1089/088282403763635465
USDA–ARS (2012) USDA national nutrient database for standard reference, Release 25. USDA–ARS, Washington, DC. https://www.nal.usda.gov/fnic/selenium
Valea A, Georgescu CE (2018) Selenoproteins in human body: focus on thyroid pathophysiology. Hormones (Athens) 17(2):183–196. https://doi.org/10.1007/s42000-018-0033-5
Valdez Barillas JR, Quinn CF, Pilon-Smits EA (2011) Selenium accumulation in plants–phytotechnological applications and ecological implications. Int J Phytoremediation 13. https://doi.org/10.1080/15226514.2011.568542
Van Hoewyk D (2013) A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann Bot 112(6):965–972. https://doi.org/10.1093/aob/mct163
Vinceti M, Filippini T, Wise LA (2018) Environmental Selenium and Human Health: an Update. Curr Environ Health Rep 5(4):464–485. https://doi.org/10.1007/s40572-018-0213-0
Wan J, Zhang M, Adhikari B (2018) Advances in selenium-enriched foods: from the farm to the fork. Trends Food Sci Tech 76:1–5. https://doi.org/10.1155/2018/8749345
Wang P, Wang H, Liu Q, Tian X, Shi Y, Zhang X (2017) QTL mapping of selenium content using a RIL population in wheat. PLoS One 12(9):e0184351. https://doi.org/10.1371/journal.pone.0184351
Wang J, Cappa JJ, Harris JP, Edger PP, Zhou W, Pires JC, Adair M, Unruh SA, Simmons MP, Schiavon M, Pilon-Smits EAH (2018) Transcriptome-wide comparison of selenium hyperaccumulator and nonaccumulator Stanleya species provides new insight into key processes mediating the hyperaccumulation syndrome. Plant Biotechnol J. https://doi.org/10.1111/pbi.12897
Wang M, Yang W, Zhou F, Du Z, Xue M, Chen T, Liang D (2019) Effect of phosphate and silicate on selenite uptake and phloem-mediated transport in tomato (Solanum lycopersicum L.). Environ Sci Pollut Res Int 26(20):20475–20484. https://doi.org/10.1007/s11356-019-04717-x
Wen H, Carignan J (2007) Reviews on atmospheric selenium: Emissions, speciation and fate. Atmos Environ 41(34):7151–7165. https://doi.org/10.1016/j.atmosenv.2007.07.035
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55(404):1927–1937. https://doi.org/10.1093/jxb/erh192
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182(1):49–84. https://doi.org/10.1111/j.1469-8137.2008.02738.x
White PJ (2016) Selenium accumulation by plants. Ann Bot 117:217–235. https://doi.org/10.1093/aob/mcv180
White PJ (2018) Selenium metabolism in plants. Biochim Biophys Acta Gen Subj S0304-4165(18)30138-7. https://doi.org/10.1016/j.bbagen.2018.05.006
WHO (2009) Global health risks: Mortality and burden of disease attributable to selected major risks. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_annex.pdf
Winkel LH, Vriens B, Jones GD, Schneider LS, Pilon-Smits E, Bañuelos GS (2015) Selenium cycling across soil-plant-atmosphere interfaces: a critical review. Nutrients 7(6):4199–4239. https://doi.org/10.3390/nu7064199
Wu ZL, Yin XB, Lin ZQ, Bañuelos GS, Yuan LX, Liu Y, Li M (2014) Inhibitory effect of selenium against Penicillium expansum and its possible mechanisms of action. Curr Microbiol 69(2):192–201. https://doi.org/10.1007/s00284-014-0573-0
Wu Z, Bañuelos GS, Lin ZQ, Liu Y, Yuan L, Yin X, Li M (2015) Biofortification and phytoremediation of selenium in China. Front Plant Sci 6:136. https://doi.org/10.3389/fpls.2015.00136
Xu J, Jia W, Hu C, Nie M, Ming J, Cheng Q, Cai M, Sun X, Li X, Zheng X, Wang J, Zhao X (2020) Selenium as a potential fungicide could protect oilseed rape leaves from Sclerotinia sclerotiorum infection. Environ Pollut 257:113495. https://doi.org/10.1016/j.envpol.2019.113495
Yao X, Chu J, Wang G (2009) Effects of selenium on wheat seedlings under drought stress. Biol Trace Elem Res 130(3):283–290. https://doi.org/10.1007/s12011-009-8328-7
Yasin M, El-Mehdawi AF, Jahn CE, Anwar A, Turner MFS, Faisal M, Pilon-Smits EAH (2015a) Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant Soil 386:385–394. https://doi.org/10.1007/s11104-014-2270-y
Yasin M, El-Mehdawi AF, Anwar A, Pilon-Smits EAH, Faisal M (2015b) Microbial-enhanced selenium and iron biofortification of wheat (Triticum aestivum L.): Applications in phytoremediation and biofortification. Int J Phytorem 17:341–347. https://doi.org/10.1080/15226514.2014.922920
Zahedi SM, Abdelrahman M, Hosseini MS, Hoveizeh NF, Tran LP (2019) Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ Pollut 253:246–258. https://doi.org/10.1016/j.envpol.2019.04.078
Zembala M, Filek M, Walas S, Mrowiec H, Kornas A, Miszalski Z, Hartikainen H (2010) Effect of selenium on macro and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil 329:457–468. https://doi.org/10.1007/s11104-009-0171-2
Zhang L, Byrne PF, Pilon-Smits EAH (2006) Mapping quantitative trait loci associated with selenate tolerance in Arabidopsis thaliana. New Phytol 170(1):33–42. https://doi.org/10.1111/j.1469-8137.2006.01635.x
Zhang L, Hu B, Li W, Che R, Deng K, Li H, Yu F, Ling H, Li Y, Chu C (2014) OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol 201(4):1183–1191. https://doi.org/10.1111/nph.12596
Zhang L, Hu B, Deng K, Gao X, Sun G, Zhang Z, Li P, Wang W, Li H, Zhang Z, Fu Z, Yang J, Gao S, Li L, Yu F, Li Y, Ling H, Chu C (2019) NRT1.1B improves selenium concentrations in rice grains by facilitating selenomethinone translocation. Plant Biotechnol J 17(6):1058–1068. https://doi.org/10.1111/pbi.13037
Zhang L, Liu Y (2020) Potential interventions for novel coronavirus in China: A systematic review. J Med Vir 92:479–490. https://doi.org/10.1002/jmv.25707
Zhao FJ, McGrath SP (2009) Biofortification and phytoremediation. Curr Opin Plant Biol 12(3):373–380. https://doi.org/10.1016/j.pbi.2009.04.005
Zhao FJ, Ago Y, Mitani N, Li RY, Su YH, Yamaji N, McGrath SP, Ma JF (2010a) The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol 186(2):392–399. https://doi.org/10.1111/j.1469-8137.2010.03192.x
Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF (2010b) Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiol 153:1871–1877. https://doi.org/10.1104/pp.110.157867
Zhu YG, Pilon-Smits EA, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14(8):436–442. https://doi.org/10.1016/j.tplants.2009.06.006
Zou C, Du Y, Rashid A, Ram H, Savasli E, Pieterse PJ, Ortiz-Monasterio I, Yazici A, Kaur C, Mahmood K, Singh S, Le Roux MR, Kuang W, Onder O, Kalayci M, Cakmak I (2019) Simultaneous biofortification of wheat with zinc, iodine, selenium, and iron through foliar treatment of a micronutrient cocktail in six countries. J Agric Food Chem 67(29):8096–8106. https://doi.org/10.1021/acs.jafc.9b01829
Acknowledgements
The authors thank Prof. Elizabeth Pilon-Smits for the critical reading of the manuscript.
Funding
Open access funding provided by Università della Calabria within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
MS had the idea for the article; MS, AE, SN, and FDV performed the literature search and data analysis; MS drafted the ms; all the authors critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Ismail Cakmak.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schiavon, M., Nardi, S., dalla Vecchia, F. et al. Selenium biofortification in the 21st century: status and challenges for healthy human nutrition. Plant Soil 453, 245–270 (2020). https://doi.org/10.1007/s11104-020-04635-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04635-9