Abstract
Background and aims
The Shaker AKT1-like channels are considered to be involved in both high- and low-affinity K+ uptake and correlated with salt tolerance in glycophytes. Suaeda salsa (Suaeda maritima subsp. salsa), as a typical salt-accumulating halophyte, is able to absorb K+ efficiently while growing under saline conditions and taking in a large amount of Na+, thus maintaining the K+ concentration in its cells. In this study, the possible functions of the inward-rectifying K+ channel SsAKT1 in K+ uptake and salt tolerance in the halophyte S. salsa were investigated.
Methods
SsAKT1 from S. salsa was isolated by RT-PCR and characterized using yeast complementation; the responses of SsAKT1 to various KCl and NaCl treatments were investigated by real-time quantitative PCR.
Results
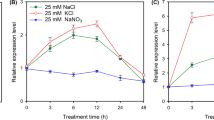
SsAKT1 consisted of 879 amino acid residues and shared high homology (60–67 %) with the identified inward-rectifying K+ channels AKT1 from other plants. The expression of SsAKT1 rescued the K+-uptake-defective phenotype of yeast strain CY162, and also suppressed the salt-sensitive phenotype of yeast strain G19, suggesting SsAKT1 functioned as an inward-rectifying K+ channel. SsAKT1 was predominantly expressed in roots, and was induced significantly by K+ starvation; transcript levels increased further on resupply of K+ (0.1–10 mM for 6 h) by 62 % in 0.1 mM K+ and 144–174 % in higher K+ concentrations (1–10 mM). Interestingly, the expression level of SsAKT1 in roots was also induced significantly by short-term treatment (6 h) with NaCl concentrations (25–250 mM).
Conclusions
These results demonstrate that the inward-rectifying K+ channel SsAKT1 might mediate both high- and low-affinity K+ uptake in S. salsa, but play a greater role in the low-affinity system. Furthermore, SsAKT1 might also be involved in salt tolerance by participating in the maintenance of K+ nutrition in S. salsa under salinity.







Similar content being viewed by others
References
Alemán F, Nieves-Cordones M, Martínez V, Rubio F (2011) Root K+ acquisition in plants: the Arabidopsis thaliana model. Plant Cell Physiol 52(9):1603–1612
Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF (1992) Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 89(9):3736–3740
Anschütz U, Becker D, Shabala S (2014) Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol 171(9):670–687
Ardie SW, Liu S, Takano T (2010) Expression of the AKT1-type K+ channel gene from Puccinellia tenuiflora, PutAKT1, enhances salt tolerance in Arabidopsis. Plant Cell Rep 29(8):865–874
Bartels D, Dinakar C (2013) Balancing salinity stress responses in halophytes and non-halophytes: a comparison between Thellungiella and Arabidopsis thaliana. Funct Plant Biol 40(9):819–831
Bauer CS, Hoth S, Haga K, Philippar K, Aoki N, Hedrich R (2000) Differential expression and regulation of K+ channels in the maize coleoptile: molecular and biophysical analysis of cells isolated from cortex and vasculature. Plant J 24(2):139–145
Benito B, Haro R, Amtmann A, Cuin TA, Dreyer I (2014) The twins K+ and Na+ in plants. J Plant Physiol 171(9):723–731
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. BBA-Biomembr 1465(1–2):140–151
Boscari A, Clement M, Volkov V, Golldack D, Hybiak J, Miller AJ, Amtmann A, Fricke W (2009) Potassium channels in barley: cloning, functional characterization and expression analyses in relation to leaf growth and development. Plant Cell Environ 32(12):1761–1777
Buschmann PH, Vaidyanathan R, Gassmann W, Schroeder JI (2000) Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+ starvation in wheat root cells. Plant Physiol 122(4):1387–1397
Cao Y, Ward JM, Kelly WB, Ichida AM, Gaber RF, Anderson JA, Uozumi N, Schroeder JI, Crawford NM (1995) Multiple genes, tissue specificity, and expression-dependent modulation contribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol 109(3):1093–1106
Chen D, Yang B, Kuo T (1992) One-step transformation of yeast in stationary phase. Curr Genet 21:83–84
Chérel I (2004) Regulation of K+ channel activities in plants: from physiological to molecular aspects. J Exp Bot 55(396):337–351
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Ann Rev Plant Physiol 31(1):239–298
Corratgé-Faillie C, Jabnoune M, Zimmermann S, Véry AA, Fizames C, Sentenac H (2010) Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell Mol Life Sci 67(15):2511–2532
Cuéllar T, Pascaud F, Verdeil JL, Torregrosa L, Adam-Blondon AF, Thibaud JB, Sentenac H, Gaillard I (2010) A grapevine Shaker inward K+ channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J 51:58–69
Czempinski K, Gaedeke N, Zimmermann S, Müller-Röber B (1999) Molecular mechanisms and regulation of plant ion channels. J Exp Bot 50:955–966
Dennison KL, Robertson WR, Lewis BD, Hirsch RE, Sussman MR, Spalding EP (2001) Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol 127:1012–1019
Elumalai RP, Nagpal P, Reed JW (2002) A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell 14(1):119–131
Epstein E, Rains D, Elzam O (1963) Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci U S A 49(5):684
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Flowers TJ, Colmer TD (2015) Plant salt tolerance: adaptations in halophytes. Ann Bot 115(3):327–331
Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Annu Rev Plant Physiol Plant Mol Biol 28:89–121
Fuchs I, Stolzle S, Ivashikina N, Hedrich R (2005) Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta 221(2):212–221
Gambale F, Uozumi N (2006) Properties of Shaker-type potassium channels in higher plants. J Membr Biol 210(1):1–19
Gierth M, Mäser P (2007) Potassium transporters in plants-Involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett 581(12):2348–2356
Gierth M, Mäser P, Schroeder JI (2005) The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 137(3):1105–1114
Golldack D, Quigley F, Michalowski CB, Kamasani UR, Bohnert HJ (2003) Salinity stress-tolerant and -sensitive rice (Oryza sativa L.) regulate AKT1-type potassium channel transcripts differently. Plant Mol Biol 51(1):71–81
Grabov A (2007) Plant KT/KUP/HAK potassium transporters: single family-multiple functions. Ann Bot 99(6):1035–1041
Hartje S, Zimmermann S, Klonus D, Mueller-Roeber B (2000) Functional characterisation of LKT1, a K+ uptake channel from tomato root hairs, and comparison with the closely related potato inwardly rectifying K+ channel SKT1 after expression in Xenopus oocytes. Planta 210(5):723–731
Hirsch RE, Lewis BD, Spalding EP, Sussman MR (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280(5365):918–921
Horie T, Brodsky DE, Costa A, Kaneko T, Schiavo FL, Katsuhara M, Schroeder JI (2011) K+ transport by the OsHKT2; 4 transporter from rice with a typical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ ions. Plant Physiol 156(3):1493–1507
Kaddour R, Nasri N, M’rah S, Berthomieu P, Lachaâl M (2009) Comparative effect of potassium on K and Na uptake and transport in two accessions of Arabidopsis thaliana during salinity stress. CR Biol 332(9):784–794
Khan MA, Ungar IA, Showalter AM (2000) The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J Arid Environ 45(1):73–84
Kronzucker HJ, Britto DT (2011) Sodium transport in plants: a critical review. New Phytol 189(1):54–81
Kronzucker HJ, Coskun D, Schulze LM, Wong JR, Britto DT (2013) Sodium as nutrient and toxicant. Plant Soil 369(1–2):1–23
Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C (1996) Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 9(2):195–203
Lebaudy A, Véry AA, Sentenac H (2007) K+ channel activity in plants: genes, regulations and functions. FEBS Lett 581(12):2357–2366
Leigh R, Wyn Jones R (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol 97(1):1–13
Li X, Zhang X, Song J, Fan H, Feng G, Wang BS (2011) Accumulation of ions during seed development under controlled saline conditions of two Suaeda salsa populations is related to their adaptation to saline environments. Plant Soil 341(1–2):99–107
Li J, Long Y, Qi GN, Li J, Xu ZJ, Wu WH, Wang Y (2014) The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 26(8):3387–3402
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Maathuis FJ (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12(3):250–258
Maathuis FJ, Amtmann A (1999) K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann Bot 84(2):123–133
Maathuis FJ, Sanders D (1994) Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc Natl Acad Sci U S A 91(20):9272–9276
Maathuis FJ, Ichida AM, Sanders D, Schroeder JI (1997) Roles of higher plant K+ channels. Plant Physiol 114(4):1141
Martinez-Cordero MA, Martinez V, Rubio F (2005) High-affinity K+ uptake in pepper plants. J Exp Bot 56(416):1553–1562
Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126(4):1646–1667
Mori S, Akiya M, Yamamura K, Murano H, Arao T, Kawasaki A, Higuchi K, Maeda Y, Yoshiba M, Tadano T (2010) Physiological role of sodium in the growth of the halophyte Suaeda salsa (L.) Pall. under high-sodium conditions. Crop Sci 50(6):2492–2498
Mori S, Suzuki K, Oda R, Higuchi K, Maeda Y, Yoshiba M, Tadano T (2011) Characteristics of Na+ and K+ absorption in Suaeda salsa (L.) Pall. Soil Sci Plant Nutr 57(3):377–386
Mumberg D, Müller R, Funk M (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156(1):119–122
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25(2):239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nieves-Cordones M, Alemán F, Martínez V, Rubio F (2014) K+ uptake in plant roots. The systems involved, their regulation and parallels in other organisms. J Plant Physiol 171(9):688–695
Pilot G, Pratelli R, Gaymard F, Meyer Y, Sentenac H (2003) Five-group distribution of the Shaker-like K+ channel family in higher plants. J Mol Evol 56:418–434
Pyo YJ, Gierth M, Schroeder JI, Cho MH (2010) High-affinity K+ Transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol 153(2):863–875
Quintero FJ, Garciadeblas B, Rodríguez-Navarro A (1996) The SAL1 gene of Arabidopsis, encoding an enzyme with 3′(2′), 5′-bisphosphate nucleotidase and inositol polyphosphate 1-phosphatase activities, increases salt tolerance in yeast. Plant Cell 8(3):529–537
Rodríguez-Navarro A, Ramos J (1984) Dual system for potassium transport in Saccharomyces cerevisiae. J Bacteriol 159(3):940–945
Römheld V, Kirkby EA (2010) Research on potassium in agriculture: needs and prospects. Plant Soil 335(1–2):155–180
Ros R, Lemaillet G, Fonrouge A, Daram P, Enjuto M, Salmon J, Thibaud J, Sentenac H (1999) Molecular determinants of the Arabidopsis AKT1 K+ channel ionic selectivity investigated by expression in yeast of randomly mutated channels. Physiol Plant 105(3):459–468
Rubio F, Nieves-Cordones M, Aleman F, Martinez V (2008) Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol Plant 134(4):598–608
Santa-María GE, Danna CH, Czibener C (2000) High-affinity potassium transport in barley roots. Ammonium-sensitive and-insensitive pathways. Plant Physiol 123(1):297–306
Schachtman DP (2000) Molecular insights into the structure and function of plant K+ transport mechanisms. BBA-Biomembr 1465(1–2):127–139
Schachtman DP, Liu WH (1999) Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci 4(7):281–287
Schroeder JI, Ward JM, Gassmann W (1994) Perspectives on the physiology and structure of inward rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol 23(1):441–471
Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C (1992) Cloning and expression in yeast of a plant potassium ion transport system. Science 256(5057):663–665
Shabala S (2003) Regulation of potassium transport in leaves: from molecular to tissue level. Ann Bot 92(5):627–634
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133(4):651–669
Shao Q, Zhao C, Han N, Wang BS (2008) Cloning and expression pattern of SsHKT1 encoding a putative cation transporter from halophyte Suaeda salsa. DNA Seq 19(2):106–114
Shao Q, Han N, Ding T, Zhou F, Wang BS (2014) SsHKT1;1 is a potassium transporter of the C3 halophyte Suaeda salsa that is involved in salt tolerance. Funct Plant Biol 41(8):790–802
Song J, Wang BS (2014) Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann Bot. doi:10.1093/aob/mcu194
Song J, Fan H, Zhao Y, Jia Y, Du X, Wang BS (2008) Effect of salinity on germination, seedling emergence, seedling growth and ion accumulation of a euhalophyte Suaeda salsa in an intertidal zone and on saline inland. Aquat Bot 88(4):331–337
Song J, Chen M, Feng G, Jia Y, Wang BS, Zhang F (2009) Effect of salinity on growth, ion accumulation and the roles of ions in osmotic adjustment of two populations of Suaeda salsa. Plant Soil 314(1–2):133–141
Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD (1999) Potassium uptake supporting plant growth in the absence of AKT1 channel activity-inhibition by ammonium and stimulation by sodium. J Gen Physiol 113(6):909–918
Su H, Golldack D, Katsuhara M, Zhao C, Bohnert HJ (2001) Expression and stress-dependent induction of potassium channel transcripts in the common ice plant. Plant Physiol 125(2):604–614
Su H, Golldack D, Zhao CS, Bohnert HJ (2002) The expression of HAK-type K+ transporters is regulated in response to salinity stress in common ice plant. Plant Physiol 129(4):1482–1493
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596–1599
Uozumi N, Nakamura T, Schroeder JI, Muto S (1998) Determination of transmembrane topology of an inward-rectifying potassium channel from Arabidopsis thaliana based on functional expression in Escherichia coli. Proc Natl Acad Sci U S A 95:9773–9778
Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI (2000) The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol 122(4):1249–1259
Véry AA, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54(1):575–603
Véry AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H (2014) Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J Plant Physiol 171(9):748–769
Volkov V, Amtmann A (2006) Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+/Na+ homeostasis under salinity stress. Plant J 48(3):342–353
Wang Y, Wu WH (2013) Potassium transport and signaling in higher plants. Annu Rev Plant Biol 64:451–476
Wang SM, Zheng WJ, Ren JZ, Zhang CL (2002) Selectivity of various types of salt-resistant plants for K+ over Na+. J Arid Environ 52:457–472
Wang SM, Zhao GQ, Gao YS, Tang ZC, Zhang CL (2004) Puccinellia tenuiflora exhibits stronger selectivity for K+ over Na+ than wheat. J Plant Nutr 27(10):1841–1857
Wang SM, Zhang JL, Flowers TJ (2007) Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiol 145(2):559–571
Ward JM, Mäser P, Schroeder JI (2009) Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol 71:59–82
Xu J, Tian X, Eneji AE, Li Z (2014) Functional characterization of GhAKT1, a novel Shaker-like K+ channel gene involved in K+ uptake from cotton (Gossypium hirsutum). Gene 545(1):61–71
Yang C, Shi D, Wang D (2008) Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regul 56(2):179–190
Yeo AR (1981) Salt tolerance in the halophyte Suaeda maritima (L.) Dum.: intracellular compartmentation of ions. J Exp Bot 32:487–497
Yeo AR, Flowers TJ (1980) Salt tolerance in the halophyte Suaeda maritima (L.) Dum.: evaluation of the effect of salinity upon growth. J Exp Bot 31:1171–1183
Zhang JL (2008) Low-affinity Na+ uptake and accumulation in the halophyte Suaeda maritima. Dissertation, Lanzhou University
Zhang JL, Shi HZ (2013) Physiological and molecular mechanisms of plant salt tolerance. Photosynth Res 115:1–22
Zhang JL, Flowers TJ, Wang SM (2010) Mechanisms of sodium uptake by roots of higher plants. Plant Soil 326:45–60
Zhang JL, Flowers TJ, Wang SM (2013) Differentiation of low-affinity Na+ uptake pathways and kinetics of the effects of K+ on Na+ uptake in the halophyte Suaeda maritima. Plant Soil 368:629–640
Zhao KF, Hai F, Ungar I (2002) Survey of halophyte species in China. Plant Sci 163(3):491–498
Zhao KF, Song J, Feng G, Zhao M, Liu JP (2011) Species, types, distribution, and economic potential of halophytes in China. Plant Soil 342(1–2):495–509
Acknowledgments
We are very grateful to Professor Alonso Rodríguez-Navarro from Centro de Biotechnología y Genómica de Plantas, Universidad Politécnica de Madrid, Spain, for providing Saccharomyces cerevisiae strain G19. We are also very grateful to Professor Timothy J. Flowers from University of Sussex, UK, for critically reviewing the manuscript and for valuable suggestions. This work was supported by the National Basic Research Program of China (973 Program, grant No. 2014CB138701), the National Natural Science Foundation of China (grant No. 31170431), Specialized Research Fund for the Doctoral Program of Higher Education of China (grant No. 20130211130001), and the Fundamental Research Funds for the Central Universities (lzujbky-2014-m01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Frans J.M Maathuis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Nucleotide sequences and deduced amino acid residues of the SsAKT1. The nucleotide sequences and amino acid residues are indicated by numbers of the left. The start codon (ATG) and the stop codon (TAA) are underlined (GIF 325 kb)
Supplementary Fig. S2
Phylogenetic groups of SsAKT1 and AKT1 from plants. The phylogenetic tree was generated by MEGA 6.0 software using the neighbour-joining method and 1000 bootstrap replicates. Bootstrap values (as percentages) are indicated at the corresponding nodes. The scale bar corresponds to a distance of 10 changes per 100 amino acid positions. SsAKT1 is shown as ●. Sources of AKT1 and their GenBank accession numbers are as follows: MKT1 (Mesembryanthemum crystallinum, AF267753), VvK1.2 (Vitis vinifera, FR669116), GmAKT1 (Glycine max, XP_003549784), NtAKT1 (Nicotiana tomentosiformis, XP_009619489), PutAKT1 (Puccinellia tenuiflora, GU327382), TaAKT1 (Triticum aestivum, AF207745), AtAKT1 (Arabidopsis thaliana, NM_128222), OsAKT1 (Oryza sativa, Os01g45990), ZMK1 (Zea mays, CAA68912), HvAKT1 (Hordeum vulgare, DQ465922) and ZxAKT1 (Zygophyllum xanthoxylum, GQ857474). (GIF 19 kb)
Supplementary Table S1
Primer sequences used in this study (DOC 37 kb)
Supplementary Table S2
The data of △Ct value corresponding to the expression data in Fig. 6 (DOC 62 kb)
Supplementary Table S3
The data of △Ct value corresponding to the expression data in Fig. 7 (DOC 29.5 kb)
Rights and permissions
About this article
Cite this article
Duan, HR., Ma, Q., Zhang, JL. et al. The inward-rectifying K+ channel SsAKT1 is a candidate involved in K+ uptake in the halophyte Suaeda salsa under saline condition. Plant Soil 395, 173–187 (2015). https://doi.org/10.1007/s11104-015-2539-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2539-9