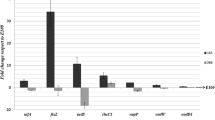
Abstract
Background and aims
Burkholderia tuberum STM678T was isolated from a South African legume, but did not renodulate this plant. Until a reliable host is found, studies on this and other interesting beta-rhizobia cannot advance. We investigated B. tuberum STM678T’s ability to induce Fix+ nodules on a small-seeded, easy-to-propagate legume (Macroptilium atropurpureum). Previous studies demonstrated that B. tuberum elicited either Fix- or Fix+ nodules on siratro, but the reasons for this difference were unexplored.
Methods
Experiments to promote effective siratro nodule formation under different environmental conditions were performed. B. tuberum STM678T’s ability to withstand high temperatures and desiccation was checked as well as its potential for promoting plant growth via mechanisms in addition to nitrogen fixation, e.g., phosphate solubilization and siderophore production. Potential genes for these activities were found in the sequenced genomes.
Results
Higher temperatures and reduced watering resulted in reliable, effective nodulation on siratro. Burkholderia spp. solubilize phosphate and produce siderophores. Genes encoding proteins potentially involved in these growth-promoting activities were detected and are described.
Conclusions
Siratro is an excellent model plant for B. tuberum STM678T. We identified genes that might be involved in the ability of diazotrophic Burkholderia species to survive harsh conditions, solubilize phosphate, and produce siderophores.







Similar content being viewed by others
Abbreviations
- hpi:
-
hours post-inoculation
- dpi:
-
days post-inoculation
- CAS:
-
chrome azurol S
References
Ahmed B, Quilt P (1980) Effect of soil moisture stress on yield, nodulation and nitrogenase activity of Macroptilium atropurpureum cv. Siratro and Desmodium intortum cv. Greenleaf. Plant Soil 57:187–194
Andrews SC, Robinson AK, Rodriguez-Quiñones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237
Boone CM, Olsthoorn MMM, Dakora FD, Spaink HP, Thomas-Oates JE (1999) Structural characterization of lipo-oligosaccharides isolated from Bradyrhizobium aspalati, microsymbionts of commercially important South African legumes. Carbohyd Res 317:155–163
Brigham RD, Hoover MM (1956) A scarifying cup for small lots of legume seed. Agron J 48:531–532
Caballero-Mellado J, Martínez-Aquilar L, Paredes-Valdez G, Estrada-de los Santos P (2004) Burkholderia unamae sp. nov., an N2-fixing rhizospheric and endophytic species. Int J System Evol Microbiol 54:1165–1172
Caballero-Mellado J, Onofre-Lemus J, Estrada-de Los Santos P, Martínez-Aguilar L (2007) The tomato rhizosphere, an environment rich in nitrogen-fixing Burkholderia species with capabilities of interest for agriculture and bioremediation. Appl Environ Microbiol 73:5308–5319
Castro-González R, Martínez-Aquilar L, Ramírez-Trujillo A, Estrada-de los Santos P, Caballero-Mellado J (2011) High diversity of culturable Burkholderia species associated with sugarcane. Plant Soil. doi:10.1007/s11104-011-07680-0
Chen W-M, Moulin L, Bontemps C, Vandamme P, Béna G, Boivin-Masson C (2003) Legume symbiotic nitrogen by β-proteobacteria is widespread in nature. J Bacteriol 185:7266–7272
Chen W-M, James EK, Coenye T, Chou J-H, Barrios E, de Faria SM, Elliott GN, Sheu SY, Sprent JI, Vandamme P (2006) Burkholderia mimosarum sp. nov., isolated from root nodules of Mimosa spp. from Taiwan and South America. Int J System Evol Microbiol 56:1847–1851
Chen W-M, de Faria SM, James EK, Elliott GN, Lin KY, Chou J-H, Sheu SY, Cnockaert M, Sprent JI, Vandamme P (2007) Burkholderia nodosa sp. nov., isolated from root nodules of the woody Brazilian legumes Mimosa bimucronata and Mimosa scabrella. Int J System Evol Microbiol 57:1055–1059
Cheng H-P, Walker GC (1998) Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J Bacteriol 180:5183–5191
Collavino M, Riccillo PM, Grasso DH, Crespi M, Aguilar OM (2005) GuaB activity is required in Rhizobium tropici during the early stages of nodulation of determinate nodules but is dispensable for the Sinorhizobium meliloti-alfalfa symbiotic interaction. Mol Plant-Microbe Inter 18:742–750
de Faria SM, de Lima HC, Olivares FL, Melo RB, Xavier RP (1999) Nodulação em espécies florestais, especificidade hospedeira e implicações na sistemática de leguminosae. In: Siqueira JO, Moreira FMS, Lopes AS, Guiherme LRG, Faquin V, Furtinia Neto AE, Carvalho JG (eds) Soil Fertility, Soil Biology, and Plant Nutrition Interrelationships. Soc Brasil Ciên Univ Fed Lavras. pp. 667–686
Dos Reis FB Jr, Simon MF, Gross E, Boddey RM, Elliott GN, Neto NE, Loureiro MF, de Queiroz LP, Scotti MR, Chen W-M, Norén A, Rubio MC, de Faria SM, Bontemps C, Goi SR, Young JP, Sprent JI, James EK (2010) Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytol 186:934–946
Elliott GN, Chen W-M, Bontemps C, Chou J-H, Young JPW, Sprent JI, James EK (2007a) Nodulation of Cyclopia spp. (Leguminosae, Papilionoideae) by Burkholderia tuberum. Ann Bot 100:1403–1411
Elliott GN, Chen W-M, Chou J-H, Wang H-C, Sheu SY, Perin L, Reis VM, Moulin L, Simon MF, Bontemps C, Sutherland JM, Bessi R, de Faria SM, Trinick MJ, Prescott AR, Sprent JI, James EK (2007b) Burkholderia phymatum is a highly effective nitrogen-fixing symbiont of Mimosa spp. and fixes nitrogen ex planta. New Phytol 173:168–180
Estévez J, Soria Díaz ME, Fernández de Córdoba F, Móron B, Manyan H, Gil A, Thomas-Oates J, van Brussel AAN, Dardanelli MS, Sousa C, Megías M (2009) Different and new Nod factors produced by Rhizobium tropici CIAT899 following Na+ stress. FEMS Microbiol Lett 293:220–231
Estrada-de los Santos P, Vacaseydel-Aceves NB, Martínez-Aquilar L, Cruz-Hernández MA, Mendoza-Herrera A, Caballero-Mellado J (2011) Cupriavidus and Burkholderia species associated with agricultural plants that grow in alkaline soils. J Microbiol 49:867–876
Fujishige NA, Lum MR, De Hoff PL, Whitelegge JP, Faull KF, Hirsch AM (2008) Rhizobium common nod genes are required for biofilm formation Mol. Microbiology 67:504–515
Garau G, Yates RJ, Deiana P, Howieson JG (2009) Novel strains of nodulating Burkholderia have a role in nitrogen fixation with papilionoid herbaceous legumes adapted to acid, infertile soils. Soil Biol Biochem 41:125–134
Graham PH, Viteri SE, Mackie F, Vargas AAT, Palacios A (1982) Variation in acid soil tolerance among strains of Rhizobium phaseoli. Field Crops Res 5:121–128
Graham PH, Draeger KJ, Ferrey ML, Conroy MJ, Hammer BE, Martínez E, Arons SR, Quinto C (1994) Acid pH tolerance in strains of Rhizobium and Bradyrhizobium and initial studies on the basis for acid tolerance of Rhizobium tropici UMR1899. Can J Microbiol 40:189–207
Gyaneshwar P, Hirsch AM, Moulin L, Chen WM, Elliott GN, Bontemps C, Estrada-de los Santos P, Gross E, dos Reis Junior FB, Sprent JI, Young JPW, James EK (2011) Legume nodulating β-proteobacteria: diversity, host range and future prospects. Mol Plant-Microbe Inter 24:1276–1288
Hernandez-Lucas L, Segovia L, Martinez-Romero E, Pueppke SG (1995) Phylogenetic relationships and host range of Rhizobium spp. that nodulate Phaseolus vulgaris L. Appl Environ Microbiol 61:2775–2779
Herridge DF, Roughley RJ (1976) Influence of temperature and Rhizobium strain on nodulation and growth of two tropical legumes. Trop Grass 10:21–23
Hirsch AM (1992) Tansley review No. 40. Developmental biology of legume nodulation. New Phytol 122:211–237
Hugenholtz P, Cunningham MA, Hendrlkz JK, Fuerst JA (1995) Desiccation resistance of bacteria isolated from an air-handling system biofilm determined using a simple quantitative membrane filter method. Lett Appl Micro 21:41–46
Iturriaga G, Suárez R, Nova-Franco B (2009) Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci 10:3793–3810
Lee HK, LaRue TA (1992) Exogenous ethylene inhibits nodulation of Pisum sativum L. cv Sparkle. Plant Physiol 100:1759–1763
Lima AS, Nobrega RSA, Barberi A, da Silva K, Ferreira DF, Moreira FMS (2009) Nitrogen-fixing bacteria communities occurring in soils under different uses in the Western Amazon Region as indicated by nodulation of siratro (Macroptilium atropurpureum). Plant Soil 319:127–145
Martínez-Romero E, Segovia L, Mercant FM, Franco AA, Graham P, Pardon MA (1991) Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Evol Microbiol 41:417–426
Mathew JA, Tan YP, Srinivasa Rao PS, Lim TM, Leung KY (2001) Edwardisella tarda mutants defective in siderophore production, motility, serum resistance and catalase activity. Microbiology 149:449–457
Michiels J, Verreth C, Vanderleyden J (1994) Effect of temperature stress on bean-nodulating Rhizobium strains. Appl Environ Microbiol 60:1206–1212
Mietzner TA, Morse SA (1994) The role of iron-binding proteins in the survival of pathogenic bacteria. Annu Rev Nutr 14:471–493
Mishra RP, Tisseyre P, Melkonian R, Chaintreuil C, Miché L, Klonowska A, Gonzalez S, Bena G, Laguerre G, Moulin L (2012) Genetic diversity of Mimosa pudica rhizobial symbionts in soils of French Guiana: investigating the origin and diversity of Burkholderia phymatum and other beta-rhizobia. FEMS Microbiol Ecol 79:487–503
Móron B, SoriaDíaz ME, Ault J, Verroios G, Noreen S, Rodríguez-Navarro DN, Gil-Serrano A, Thomas-Oates J, Megías M, Sousa C (2005) Low pH changes the profile of nodulation factors produced by Rhizobium tropici CIAT899. Chem Biol 12:1020–1040
Moulin L, Munive A, Dreyfus B, Boivin-Masson C (2001) Nodulation of legumes by members of the beta-subclass of proteobacteria. Nature 411:948–950
Muofhe ML, Dakora FD (1998) Bradyrhizobium species isolated from indigenous legumes of the Western Cape exhibit high tolerance of low pH. In: Elmerich C, Kondorosi A, Newton WE (eds) Biological nitrogen fixation for the 21st century. Kluwer Academic Publishers, Dordrecht, p 519
Ohashi Y, Saneoka H, Fujita K (2000) Effect of water stress of growth, photosynthesis, and photoassimilate translocation in soybean and tropical pasture legume siratro. Soil Sci Plant Nutr 46:417–425
Perin L, Martinez-Aquilar L, Paredes-Valdez G, Baldani JI, Estrada-de Los Santos P, Reis VM, Cabellero-Mellado J (2006) Burkholderia silvatlantica sp. nov., a bacterium associated with field-grown sugarcane and maize. Int J Syst Evol Microbiol 56:1931–1937
Pikovskaya RI (1948) Mobilization of phosphorous soil in connection with the vital activity of some microbial species. Microbiol (Mikrobiol) 17:362–370
Pinto FGS, Chueire LMO, Vasconcelos ATR, Nicolás MF, Almeida LGP, Souza RC, Menna P, Barcellos FG, Megías N, Hungria M (2009) Novel genes related to nodulation, secretion systems, and surface structures revealed by a genome draft of Rhizobium tropici strain PRF 81. Funct Integr Genom 9:263–270
Pueppke SG, Broughton WJ (1999) Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant-Microbe Inter 12:293–318
Riccillo PM, Collavino MM, Grasso DH, England R, de Bruijn FJ, Aguilar OM (2000) A guaB mutant strain of Rhizobium tropici CIAT899 pleiotropically defective in thermal tolerance and symbiosis. Mol Plant-Microbe Inter 13:1228–1236
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rodríguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 287:15–21
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sheriff DW, Ludlow MM (1984) Physiological reactions to an imposed drought by Macroptilium atropurpureum and Cenchrus ciliaris in a mixed sward. Aust J Plant Physiol 11:23–34
Sprent JI (2007) Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytol 186:934–946
Streeter JG, Gomez ML (2006) Three enzymes for trehalose synthesis in Bradyrhizobium cultured bacteria and in bacteroids from soybean nodules. Appl Environ Microbiol 72:4250–4255
Suárez-Moreno AR, Cabellero-Mellado J, Coutinho BG, Mendonça-Previato L, James EK, Venturi V (2012) Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb Ecol 63:249–266
Suzuki A, Suriyagoda L, Shigeyama T, Tominaga A, Sasaki M, Hiratsuka Y, Yoshinaga A, Arima S, Agarie S, Sakai T, Inada S, Jikumaru Y, Kamiya Y, Uchiumi T, Abe M, Hashiguchi M, Akashi T, Sato S, Kaneko T, Tabata S, Hirsch AM (2011) Lotus japonicus nodulation is photomorphogenetically controlled by sensing the R/FR ratio through JA signaling. Proc Natl Acad Sci USA 108:16837–16842
Van Brussel AAN, Tak T, Wetselaar A, Pees E, Wijffelman CA (1982) Small Leguminosae as test plants for nodulation of Rhizobium leguminosarum and other rhizobia an agrobacteria harbouring a leguminosarum sym-plasmid. Plant Sci Lett 27:317–325
Vandamme P, Goris J, Chen W-M, de Vos P, Willems A (2002) Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov., nodulate the roots of tropical legumes. Syst Appl Microbiol 25:507–512
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications, London
Weisskofp L, Heller S, Eberl L (2011) Burkholderia species are major inhabitants of white lupin cluster roots. Appl Environ Microbiol 77:7715–7720
Xie J, Knight D, Legget M (2009) Comparison of media used to evaluate Rhizobium leguminosarum biovar viciae for phosphate-solubilizing ability. Can J Microbiol 55:910–915
Yao W, Byrne R (2001) Spectrophotometric determination of freshwater pH using bromocresol purple and phenol red. Environ Sci Technol 35:1197–1201
Yeung S-L, Cheng C, Lui TKO, Tsang JSH, Chan W-T, Lim BL (2009) Purple acid phosphastase-like sequences in prokaryotic genomes and the characterization of an atypical purple alkaline phosphatase from Burkholderia cenocepacia J2315. Gene 440:1–8
Acknowledgments
This research was supported in part by a grant (IOB-0537497) from the National Science Foundation (USA) to GW and AMH and a Shanbrom Family Foundation grant to AMH. A University of California Office of The President, President’s Postdoctoral Fellowship, supported AA. We thank the National Germplasm Collection of the USDA-ARS for seeds of various cowpea varieties that were tested and the Joint Genome Institute/Department of Energy for the annotation platform. LMU’s Seaver College of Science and Engineering M.A.N.E. laboratory is thanked for the use of the confocal microscope.
Liamara Perin and Veronica M. Reis of EMBRAPA are acknowledged for their previous research on Burkholderia species and for providing helpful information regarding B. silvatlantica SRMrh20T. J. Peter Young is thanked for providing the B. tuberum/gfp strain. Members of the Hirsch laboratory, especially Drs. Drora Kaplan and Nisha Tak are thanked for reviewing the manuscript.
This paper is dedicated to the memory of Jesus Cabellero-Mellado, one of the pioneers in the study of the plant-associated Burkholderia species.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Burkholderia tuberum STM678T-inoculated plants nodulate under water-stressed conditions. Dishpans containing siratro plants inoculated (a and c) with B. tuberum STM678T or left uninoculated (b). Dishpans a) and b) were watered with ¼ strength Hoagland’s medium minus N twice weekly whereas dishpan c was watered every other week. (JPEG 398 kb)
Supplementary Figure 2
Burkholderia species vary in ability to solubilize inorganic phosphate depending on the supplied carbon source. (a) When either sucrose or glucose was supplied as the sole carbon source in the preparation of PVK medium, a difference in the ability to solubilize inorganic phosphate was observed on agar plates. (b) Measurements indicate the zone of clearance (if any) calculated by measuring the surrounding halo to equal starting OD values of bacteria. Error bars indicate standard error. (JPEG 242 kb)
Supplementary Figure 3
Burkholderia species secrete siderophores. (a) Iron acquisition via siderophore secretion was detected 5 dpi using chrome azurol S (CAS) overlay agar assay plates for each of the Burkholderia species studied. The presence of the yellow/orange halo around the spot of bacterial growth indicates the presence of iron-binding siderophores being secreted into the surrounding media. (b) Measurements indicate the zone of each halo, calculated by taking the ratio of the surrounding halo to that of the bacterial growth. Error bars indicate standard error. (JPEG 7 kb)
Rights and permissions
About this article
Cite this article
Angus, A.A., Lee, A., Lum, M.R. et al. Nodulation and effective nitrogen fixation of Macroptilium atropurpureum (siratro) by Burkholderia tuberum, a nodulating and plant growth promoting beta-proteobacterium, are influenced by environmental factors. Plant Soil 369, 543–562 (2013). https://doi.org/10.1007/s11104-013-1590-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1590-7