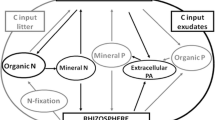
Abstract
Two strategies for phosphorus (P) economy in P-limiting environment are conservation of use and enhanced acquisition. Using two wetland macrophytes as an example, we show how these strategies change when the P-limitation is removed. Phosphorus resorption and activities of root phosphatases were evaluated over 4 years in Eleocharis cellulosa Torr. and Typha domingensis Pers. from nutrient addition experiment (P, N, N&P, control) established in 15 P limited marshes of Belize. We hypothesized that after P addition both species will increase tissue P content and decrease P resorption efficiency and root phosphatase activity. Initially high phosphorus resorption efficiency, PRE, significantly decreased in Eleocharis 2 years after the first nutrient addition, while no significant decrease was recorded for Typha. Even more dramatic was 5- to 6-fold increase in P in senescent tissues of Eleocharis as compared to less than 2-fold increase in Typha. Root phosphatase activity was high for both species from control plots. After P addition, Eleocharis showed 35% to 70% decrease in enzyme activity correlated to availability of inorganic P in sediments. Eleocharis and Typha employ the “conservation of use” strategy when growing in P limited oligotrophic marshes. In addition, Eleocharis is also using the “enhanced acquisition” strategy. These strategies change when the P limitation is removed but the response varies between the two species and thus changes in the proportion of these two species in a community may result in differences in ecosystem processes such as decomposition.






Similar content being viewed by others
References
Abel S, Ticconi CA, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115:1–8
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Asmar F, Gissel-Nielsen G (1997) Extracellular phosphomono- and phosphodiesterase associated with and released by the roots of barley genotypes: a non-destructive method for the measurement of the extracellular enzymes of roots. Biol Fertil Soils 25:117–122
Boers AM, Veltman RLD, Zedler JB (2007) Typha x glauca dominance and extended hydroperiod constrain restoration of wetland diversity. Ecol Eng 29:232–244
Cai ZQ, Bongers F (2007) Contrasting nitrogen and phosphorus resorption efficiencies in trees and lianas from a tropical montane rain forest in Xishuangbanna, south-west China. J Trop Ecol 23:115–118
Chen HJ, Mendelssohn IA, Lorenzen B, Brix H, Miao SL (2005) Growth and nutrient responses of Eloecharis cellulosa (Cyperaceae) to phosphate level and redox intensity. Am J Bot 92:1457–1466
Craft CB, Richardson CJ (1993) Peat accretion and phosphorus accumulation along a eutrophication gradient in the northern Everglades. Biogeochemistry 22:133–156
Craft CB, Vymazal J, Richardson CJ (1995) Response of Everglades plant-communities to nitrogen and phosphorus additions. Wetlands 15:258–271
Davis SE, Corronado-Molina C, Childers DL, Day JW (2003) Temporally dependent C, N, and P dynamics associated with the decay of Rhizophora mangle L. leaf litter in oligotrophic mangrove wetlands of the Southern Everglades. Aquat Bot 75:199–215
Davis SE, Childers DL, Noe GB (2006) The contribution of leaching to the rapid release of nutrients and carbon in the early decay of wetland vegetation. Hydrobiologia 569:87–97
Duff SMG, Sarath G, Plaxton WC (1994) The role of acid phosphatases in plant phosphorus metabolism. Physiol Plant 90:791–800
Feller IC, Whigham DF, O'Neill JP, McKee KL (1999) Effects of nutrient enrichment on within-stand cycling in a mangrove forest. Ecology 80:2193–2205
Feller IC, McKee KL, Whigham DF, O'Neill JP (2002) Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 62:145–175
Field C (1983) Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56:341–347
Garbey C, Murphy KJ, Thiebaut G, Muller S (2004) Variation in P-content in aquatic plant tissues offers an efficient tool for determining plant growth strategies along a resource gradient. Freshw Biol 49:346–356
Grime JP (2001) Plant Strategies,Vegetation Processes, and Ecosystem Properties. Wiley, Chichester, p 417
Gűsewell S (2005) Nutrient resorption of wetland graminoids is related to the type of nutrient limitation. Funct Ecol 19:344–354
Gűsewell S, Koerselman M (2002) Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect Plant Ecol Evol Syst 5:37–61
Harrington RA, Fownes JH, Vitousek PM (2001) Production and resource use efficiencies in N- and P limited tropical forests: a comparison of responses to long-term fertilization. Ecosystems (N Y, Print) 4:646–657
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hoppe HG (1993) Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria. In: Kemp PF (ed) Handbook of methods in aquatic microbial ecology. Lewis Publ., Boca Raton, FL, pp 423–431
Johnson D, Leake JR, Lee JA (1999) The effects of quantity and duration of simulated pollutant nitrogen deposition on root-surface phosphatase activities in calcareous and acid grasslands: a bioassay approach. New Phytol 141:433–442
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727
King RB, Baillie IC, Abell TMB, Dunsmore JR, Gray DA, Pratt JH, et al (1992) Land Resources Assessment of Northern Belize. 513p
Kobe RK, Lepczyk CA, Iyer M (2005) Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 86:2780–2792
Kourtev PS, Ehrenfeld JG, Huang WZ (2002) Enzyme activities during litter decomposition of two exotic and two native plant species in hardwood forests of New Jersey. Soil Biol Biochem 34:1207–1218
Kroehler CJ, Linkins AE (1988) The root surface phosphatases of Eriophorum vaginatum: effects of temperature, pH, substrate concentration and inorganic phosphorus. Plant Soil 105:3–10
Kuhn NL, Mendelssohn IA, McKee KL, Lorenzen B, Brix H, Miao SL (2002) Root phosphatase activity in Cladium jamaicense and Typha domingensis grown in Everglades soil at ambient and elevated phosphorus levels. Wetlands 22:794–800
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot (Lond) 98:693–713
Macek P, Rejmánková E (2007) Response of emergent macrophytes to experimental nutrient and salinity additions. Funct Ecol 21:478–488
McKee KI, Mendelssohn IA, Hester MW (1988). Reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. Amer J Bot 75:1352–1364
McNamara AE, Hill WR (2000) UV-B irradiance gradient affects photosynthesis and pigments but not food quality of periphyton. Freshw Biol 43:649–662
Miller RO (1998) Extractable chloride, nitrate, orthophosphate, potassium, and sulfate-sulfur in plant tissue: 2% acetic acid extraction. In: Karla YP (ed) handbook of reference methods for plant analyses. CRC, New York, pp 115–118
Miao SL (2004) Rhizome growth and nutrient resorption: mechanisms underlying the replacement of two clonal species in Florida Everglades. Aquat Bot 78:55–66
Miao SL, Newman S, Sklar FH (2000) Effects of habitat nutrients and seed sources on growth and expansion of Typha domingensis. Aquat Bot 68:297–311
Newman S, Grace JB, Koebel JW (1996) Effects of nutrients and hydroperiod on Typha, Cladium, and Eleocharis: implications for Everglades restoration. Ecol Appl 6:444–783
Newman S, McCormick PV, Miao SL, Laing JA, Kennedy WC, O'Dell MB (2004) The effect of phosphorus enrichment on the nutrient status of a northern Everglades slough. Wetlands Ecol Manag 12:63–79
Noe GB, Childers DL, Jones RD (2001) Phosphorus biogeochemistry and the impact of phosphorus enrichment: why is the Everglades so unique? Ecosystems (N Y, Print) 4:603–624
Phoenix GK, Booth RE, Leake JR, Read DJ, Grime JP, Lee JA (2004) Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol 161:279–289
Playsted CWS, Johnston ME, Ramage CM, Edwards DG, Cawthray GR, Lambers H (2006) Functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol 170:491–500
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274:37–49
Rejmánková E (2001) Effect of experimental phosphorus enrichment on oligotrophic tropical marshes in Belize, Central America. Plant Soil 236:33–53
Rejmánková E (2005) Nutrient resorption in wetland macrophytes: comparison across several regions of different nutrient status. New Phytol 167:471–482
Rejmánková E, Houdková K (2006) Wetland plant decomposition under different nutrient conditions: what is more important, litter quality or site quality? Biogeochemistry 80:245–262
Rejmánková E, Pope KO, Post R, Maltby E (1996) Herbaceous wetlands of the Yucatan Peninsula: communities at extreme ends of environmental gradients. Int Rev Gesamten Hydrobiol 81:223–252
Rejmánková E, Komarek J, Komárková J (2004) Cyanobacteria—a neglected component of biodiversity: patterns of species diversity in inland marshes of northern Belize (Central America). Div Dist 10:189–199
Rejmánková E, Grieco J, Achee N, Masuoka P, Pope K, Roberts D et al (2006) Freshwater community interactions and malaria. In: Collinge SK, Ray Ch (eds) Disease Ecology, Oxford
Rejmánková E, Macek P, Kimberly Epps K (in press) Wetland ecosystem changes after three years of phosphorus addition. Wetlands
Richardson AE, George TS, Hens M, Simpson RJ (2005a) Utilization of soil organic phosphorus by higher plants. In: Turner BL, Frossard E, Baldwin DS (eds) Organic phosphorus in the environment. CABI, Cambridge, pp 165–184
Richardson SJ, Peltzer DA, Allen RB, McGlone MS (2005b) Resorption proficiency along a chronosequence: responses among communities and within species. Ecology 86:20–25
SAS Institute Inc 1998. Statview 5.0.1 Reference—SAS Institute, Inc., Cary, North Carolina
Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58:47–69
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Tarafdar JC, Claassen N (2005) Preferential utilization of organic and inorganic sources of phosphorus by wheat plant. Plant Soil 275:285–293
Ticconi CA, Abel S (2004) Short on phosphate: plant surveillance and countermeasures. Trends Plant Sci 9:548–555
Touchette BW, Burkholder JM (2007) Effects of temperature and nitrate on phosphomonoesterase activities between carbon source and sink tissues in Zostera marina L. J Exp Mar Biol Ecol 342:313–324
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:946–954
Turner BL, Newman S (2005) Phosphorus cycling in wetland soils: the importance of phosphate diesters. J Environ Qual 34:1921–1929
Turner BL, Baxter R, Ellwood NTW, Whitton BA (2001) Characterization of the phosphatase activities of mosses in relation to their environment. Plant Cell Environ 24:1165–1176
van Heerwaarden LM, Toet S, Aerts R (2003a) Current measures of nutrient resorption efficiency lead to a substantial underestimation of real resorption efficiency: facts and solutions. Oikos 101:664–669
van Heerwaarden LM, Toet S, Aerts R (2003b) Nitrogen and phosphorus resorption efficiency and proficiency in six sub-arctic bog species after 4 years of nitrogen fertilization. J Ecol 91:1060–1070
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Vitousek PM (1998) Foliar and litter nutrients, nutrient resorption, and decomposition in Hawaiian Metrosideros polymorpha. Ecosystems (N Y, Print) 1:401–407
Walk TC, Jaramillo R, Lynch JP (2006) Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 279:347–366
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophylll species. Funct Ecol 17:10–19
Acknowledgments
We thank to Russell King, Ireneo Briceno, Dagmara Sirová, and Petr Macek for their assistance in the field and to Joy and Joel Futrell for laboratory assistance. Comments of Marcel Rejmanek to the first draft of the manuscript are appreciated. Comments and suggestions of two anonymous reviewers greatly improved the manuscript. This research was supported by NSF grant # 0089211 to E.R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Rights and permissions
About this article
Cite this article
Rejmánková, E., Snyder, J.M. Emergent macrophytes in phosphorus limited marshes: do phosphorus usage strategies change after nutrient addition?. Plant Soil 313, 141–153 (2008). https://doi.org/10.1007/s11104-008-9687-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9687-0