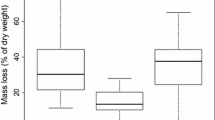
Abstract
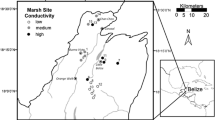
Plants in nutrient poor environments are often characterized by high nutrient resorption resulting in poor litter quality and, consequently, slow decomposition. We used oligotrophic, P-limited herbaceous wetlands of northern Belize as a model system, on which to document and explain how changes in nutrient content along a salinity gradient affect decomposition rates of macrophytes. In 2001 we established a nutrient addition experiment (P, N, and N&P) in 15 marshes of a wide range of water conductivities (200–6000 μS), dominated by Eleocharis spp. To determine what is more important for decomposition, the initial litter quality, or site differences, we used reciprocal litter placement and cellulose decomposition assay in a combined “site quality” and “litter quality” experiment. Our prediction of the positive effects of P-enrichment on decomposition rate due to both the quality of litter and the site was confirmed. The site effect was stronger than the litter quality although both were highly significant. Strong site quality effect was apparently the result of more active decomposer community in P-enriched plots as supported by finding of higher microbial biomass in litter decomposing there. The strong effect of site quality on decomposition was further confirmed by the cellulose assay. The cellulose decomposition was significantly slower at high salinity sites indicating lower decomposer microbial activity. Litter nutrient N and P content and nutrient ratios were well correlated with decomposition with the best fit found for log C/P. At C/P mass ratio of >4000 decomposition processes were extremely slow. We hypothesize that in a long run, the increased decomposition will compensate the increase in primary production resulting from increased nutrient loading and there will be no differences in accumulation of organic material between the controls and nutrient enriched plots.
Similar content being viewed by others
References
Aerst R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Aerts R, De Caluwe H (1997) Initial litter respiration as indicator for long-term leaf litter decomposition of Carex species. Oikos 80:353–361
Alvarez S, Guerrero MC (2000) Enzymatic activities associated with decomposition of particulate organic matter in two shallow ponds. Soil Biol Biochem 32:1941–1951
Ayyappan S, Olah J, Raghavan SL, Sinha VRP, Purushothaman CS (1986) Macrophyte decomposition in two tropical lakes. Arch Hydrobiol 106:219–231
Bünemann EK, Bossio DA, Smithson PC, Frossard E, Oberson A (2004) Microbial community composition and substrate use in a highly weathered soil as affected by crop rotation and P fertilization. Soil Biol Biochem 36:889–901
Canfield DE, Thamdrup B, Kristensen E (2005) Aquatic geimicrobiology. Advances in marine biology, vol 48. Elsevier, Amsterdam, p 424
Coûteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Davis SE, Corronado-Molina C, Childers DL, Day JW (2003) Temporally dependent C, N, and P dynamics associated with the decay of Rhizophora mangle L. leaf litter in oligotrophic mangrove wetlands of the Southern Everglades. Aquat Bot 75:199–215
Enriquez S, Duarte CM, Sandjensen K (1993) Patterns in decomposition rates among photosynthetic organisms – the importance of detritus C–N–P content. Oecologia 94:457–471
Feller IC, Wigham DF, O’Neill JP, McKee KL (1999) Effects of nutrient enrichment on within-stand cycling in a mangrove forest. Ecology 80:2193–2205
Gulis V, Suberkropp K (2003) Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshwater Biol 48:123–134
Hassett JE, Zak DR (2005) Aspen harvest intensity decreases microbial biomass, extracellular enzyme activity, and soil nitrogen cycling. Soil Sci Soc Am J 69:227–235
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Hobbie SE, Gough L (2004) Litter decomposition in moist acidic and non-acidic tundra with different glacial histories. Oecologia 140:113–124
Holmboe N, Kristensen E, Andersen FO (2001) Anoxic decomposition in sediments from a tropical mangrove forest and the temperate Wadden Sea: implications of N and P addition experiments. Estuar Coast Shelf Sci 53:125–140
Hoorens B, Aerts R, Stroetenga M (2003) Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 137:578–586
Hunter DA, Reuter JE, Goldman CR (1993) Standard operating procedures. In: Lake Tahoe Interagency Monitoring Program, UC Davis
Iiyama K, Wallis AFA (1989) Effect of acetyl bromide treatment on the ultraviolet-spectra of lignin model compounds. Holzforschung 43:309–316
King RB, Baillie IC, Abell TMB, Dunsmore JR, Gray DA, Pratt JH, Versey HR, Wright ACS, Zisman SA (1992) Land resources assessment of Northern Belize. Nat Resour Institute Bull 43:513
Kourtev PS, Ehrenfeld JG, Huang WZ (2002) Enzyme activities during litter decomposition of two exotic and two native plant species in hardwood forests of New Jersey. Soil Biol Biochem 34:1207–1218
Kuehn KA, Suberkropp K (1998) Diel fluctuations in rates of CO2 evolution from standing dead leaf litter of the emergent macrophyte Juncus effusus. Aquat Microb Ecol 14:171–182
Kurka AM, Starr M, Heikinheimo M, Salkinoja Salonen M (2000) Decomposition of cellulose strips in relation to climate, litterfall nitrogen, phosphorus and C/N ratio in natural boreal forests. Plant Soil 219:91–101
Kwabiah AB, Stoskopf NC, Voroney RP, Palm CA (2001) Nitrogen and phosphorus release from decomposing leaves under sub-humid tropical conditions. Biotropica 33:229–240
Liski J, Nissinen A, Erhard M, Taskinen O (2003) Climatic effects on litter decomposition from arctic tundra to tropical rainforest. Global Change Biol 9:575–584
Lovett GM, Weathers KC, Arthur MA, Schultz JC (2004) Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry 67:289–308
Maltby E (1985) Effects of nutrient loadings on decomposition profiles in the water column and submerged peat in the Everglades. In: Tropical peat resources – prospects and potential, proceedings of the international symposium of Peat Society, pp 450–464
Mendelssohn IA, Slocum MG (2004) Relationship between soil cellulose decomposition and oil contamination after an oil spill at Swanson Creek, Maryland. Mar Pollut Bull 48:359–370
Menéndez M, Hernandez O, Comin FA (2003) Seasonal comparisons of leaf processing rates in two Mediterranean rivers with different nutrient availability. Hydrobiologia 495:159–169
Morris JT, Bowden WB (1986) A mechanistic, numerical-model of sedimentation, mineralization, and decomposition for marsh sediments. Soil Sci Soc Am J 50:96–105
Morris JT, Bradley PM (1999) Effects of nutrient loading on the carbon balance of coastal wetland sediments. Limnol Oceanogr 44:699–702
Newman S, Kumpf H, Laing JA, Kennedy WC (2001) Decomposition responses to phosphorus enrichment in an Everglades (USA) slough. Biogeochemistry 54:229–250
Paterson E (2003) Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur J Soil Sci 54:741–750
Paul EA, Clark FE (1989) Soil microbiology and biochemistry. Academic Press, San Diego
Poll C, Thiede A, Wermbter N, Sessitsch A, Kandeler E (2003) Micro-scale distribution of microorganisms and microbial enzyme activities in a soil with long-term organic amendment. Eur J Soil Sci 54:715–724
Qualls RG, Richardson CJ (2003) Factors controlling concentration, export, and decomposition of dissolved organic nutrients in the Everglades of Florida. Biogeochemistry 62:197–229
Rejmánková E (2001) Effect of experimental phosphorus enrichment on oligotrophic tropical marshes in Belize, Central America. Plant Soil 236:33–53
Rejmánková E, Komárková J (2000) A function of cyanobacterial mats in phosphorus-limited tropical wetlands. Hydrobiologia 431:135–153
Rejmánková E, Komárek J, Komárková J (2004) Cyanobacteria – a neglected component of biodiversity: patterns of species diversity in inland marshes of northern Belize (Central America). Divers Distribut 10:189–199
Royer TV, Minshall GW (1997) Rapid breakdown of allochthonous and autochthonous plant material in a eutrophic river. Hydrobiologia 344:81–86
Shaver GR, Melillo JM (1984) Nutrient budgets of marsh plants – efficiency concepts and relation to availability. Ecology 65:1491–1510
Talling JF, Lemoalle J (1998) Ecological dynamics of tropical inland waters. Cambridge University Press, Cambridge
Ulehlova B (1976) Microbial decomposers and decomposition processes in wetlands. Academia, Praha
Verhoeven JTA, Keuter A, Van Logtestijn R, Van Kerkhoven MB, Wassen M (1996) Control of local nutrient dynamics in mires by regional and climatic factors: a comparison of Dutch and Polish sites. J Ecol 84:647–656
Vitousek PM (2004) Nutrient cycling and limitation: Hawai’i as a model system. Princeton University Press, Princeton, NJ
Vitousek PM, Turner DR, Parton WJ, Sanford RL (1994) Litter decomposition on the Mauna-Loa environmental matrix, Hawaii – patterns, mechanisms, and models. Ecology 75:418–429
Waterman PG, Mole S (1994) Analysis of plant metabolites. Methods in ecology series. Blackwell Scientific Publications, Oxford
Weidie AE (1985) Geology of the Yucatan Platform, part 1. In: Ward WC, Weidie AE, Back W (eds) Geology and hydrogeology of the Yucatan and quaternary geology of northeastern Yucatan Peninsula. New Orleans Geological Society, New Orleans, LA, pp 1–19
Xuluc-Tolosa FJ, Vester HFM, Ramirez-Marcial N, Castellanos-Albores J, Lawrence D (2003) Leaf litter decomposition of tree species in three successional phases of tropical dry secondary forest in Campeche, Mexico. Forest Ecol Manage 174:401–412
Acknowledgements
We thank to Petr Macek, Kate Knox, Ireneo Briceno, and Russell King for their assistance in the field, to Joy Futrell for laboratory assistance, and to Rebecca Drenovsky for PLFA analyses. We also thank to Jan Lepš for his help with the unbalanced ANOVA design and to Jenise Snyder for editorial comments. The comments of the two anonymous reviewers and the journal editor helped to improve the manuscript. This research was supported by NSF grant NSF # 0089211 to E.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rejmánková, E., Houdková, K. Wetland plant decomposition under different nutrient conditions: what is more important, litter quality or site quality?. Biogeochemistry 80, 245–262 (2006). https://doi.org/10.1007/s10533-006-9021-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-006-9021-y