Abstract
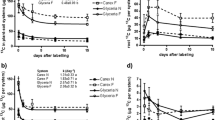
We compared exudation and rhizosphere microbial activity of two macrophytes growing in tropical marshes. Eleocharis spp. are adapted to low nutrient level in phosphorus limited conditions, while Typha domingensis is a strong competitor in nutrient enriched areas. In situ measurements of carbon fluxes from roots to interstitial water and 13C partitioning after pulse-labelling of the plants in a mesocosm experiment were used to estimate root-derived C fluxes to rhizosphere under P limited and enriched conditions. Root-released compounds collected in the field were analysed for dissolved organic C, dissolved nitrogen and their biodegradability was characterized through microbial respiration, N mineralization and phosphatase activity. Independent of P loading, Eleocharis released more C from roots than T. domingensis, and the released compounds were more biodegradable. The two species responded to P enrichment differently. While Eleocharis invested more assimilated 13C to the belowground (roots, rhizomes and rhizodepositions) after P fertilization, in T. domingensis the belowground investment decreased. The effect of plant species on belowground C allocation was larger than the effect of P enrichment. Low nutrients adapted Eleocharis invested more carbon into exudation and promotion of its rhizosphere microbial community while competitive T. domingensis spent more fixed carbon on its own growth and metabolism.



Similar content being viewed by others
References
Arrigo KR (2005) Marine microorganisms and global nutrients cycles. Nature 437:349–356
Badri DV, Vivavco JM (2009) Regulation and function of root exudates. Plant, Cell & Environment 32:666–681
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. Microbial Ecology 68:1–13
Brix H, Lorenzen B, Mendelssohn IA, McKee KL, Miao SL (2010) Can differences in phosphorus uptake kinetics explain distribution of cattail and sawgrass in the Florida Everglades? Plant Biology 10:23
Brüggemann N, Gessler A, Kayler Z, Keel SG, Badeck F, Barthel M, Boeckx BN, Brugnoli E, Esperschütz J, Gavrichkova O, Gashghaie J, Gomez-Casanovas N, Keitel C, Knohl A, Kuptz D, Palacio S, Salmon Y, Uchida Y, Bahn M (2011) Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences 8:3457–3489
Calvaruso C, Turpault MP, Frey-Klett P (2006) Root associated bacteria contribute to mineral weathering and to mineral nutrition in trees: a budgeting analysis. Applied and Environmental Microbiology 72:1258–1266
Černá B, Rejmánková E, Snyder JM, Šantrůčková H (2009) Heterotrophic nitrogen fixation in oligotrophic tropical marshes: changes after phosphorus addition. Hydrobiologia 627:55–65
Grayston SJ, Vaughan D, Jones D (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Applied Soil Ecology 5:29–56
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. The New Phytologist 164:243–266
Hill PW, Marshall C, Williams GG, Blum H, Harmens H, Jones DL, Farrar JF (2007) The fate of photosynthetically-fixed carbon in Lolium perenne grassland as modified by elevated CO2 and sward management. The New Phytologist 173:766–777
Höfle MG (1990) RNA chemotaxonomy of bacterial isolates and natural microbial communities. In: Overbeck J, RJ C (eds) Aquatic microbial ecology: biochemical and molecular approaches. Springer, New York, pp. 129–154 pp 185
Hunter PJ, Teakle G, Bending GD (2014) Root traits and microbial community interactions in relation to phosphorus availability and acquisition, with particular reference to Brassica. Frontiers in Plant Science 5:27
Johnson S, Rejmánková E (2005) Impacts of land use on nutrient distribution and vegetation composition of freshwater wetlands in Northern Belize. Wetlands 25:89–100
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. The New Phytologist 163:459–480
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant and Soil 321:5–33
Kaštovská E, Šantrůčková H (2007) Fate and dynamics of recently fixed C in pasture plant-soil system under field conditions. Plant and Soil 300:61–69
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biology and Biochemistry 42:1363–1371
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review. Journal of Plant Nutrition and Soil Science 163:421–431
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98:693–713
Lambers H, Mougel C, Jaillard B, Hinsinger P (2009) Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective. Plant and Soil 321:83–115
Leake JR, Ostle NJ, Rangel-Castro JI, Johnson D (2006) Carbon fluxes from plants through soil organisms determined by field 13CO2 pulse-labelling in an upland grassland. Applied Soil Ecology 33:152–175
Macek P, Rejmánková E, Lepš J (2010) Dynamics of Typha domingensis spread in Eleocharis dominated oligotrophic tropical wetlands following nutrient enrichment. Evolutionary Ecology 24:1505–1519
Marschner P, Yang CH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biology and Biochemistry 33:1437–1445
Marschner P, Crowley D, Rengel Z (2011) Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biology and Biochemistry 43:883–894
McCoy MB, Rodriguez JM (1994) Cattail (Typha domingensis) eradication methods in the restoration of a tropical seasonal freshwater marsh. In: Mitsch J (ed) Global wetlands: old world and new. Elsevier, New York, pp. 469–482
McNamara AE, Hill WR (2000) UV-B irradiance gradient affects photosynthesis and pigments but not food quality of periphyton. Freshwater Biology 43:649–662
Nguyen C (2003) Rhizodeposition of organic c by plants: mechanisms and controls. Agronomie 23:375–396
Oelmann Y, Richter AK, Roscher C, Rosenkranz S, Temperton VM, Weisser WW, Wilcke W (2011) Does plant diversity influence phosphorus cycling in experimental grasslands? Geoderma 167-168:178–187
Personeni E, Loiseau P (2005) Species strategy and N fluxes in grassland soil: a question of root litter quality or rhizosphere activity? European Journal of Agronomy 22:217–229
Phillippot L, Hallin S, Börjesson G, Baggs EM (2009) Biochemical cycling in the rhizosphere having an impact on global change. Plant and Soil 321:61–81
Pivničková B, Šantrůčková H, Rejmánková E, Snyder JM (2010) Heterotrophic microbial activities and nutritional status of microbial communities in tropical marsh sediments of different salinities: the effect of phosphorus additionand plant species. Plant and Soil 336:49–63
Reddy R, DeLaune RD (2008) Biogeochemistry of wetlands. Chapter 3 biogeochemical characteristics. CRC Press, Boca Raton, pp. 27–65
Rejmánková E, Snyder JM (2008) Emergent macrophytes in phosphorus limited marshes: do phosphorus usage strategies change after nutriet addition? Plant and Soil 313:141–153
Rejmánková E, Komárek J, Komárková J (2004) Cyanobacteria – a neglected component of biodiversity: patterns of species diversity of inland marshes of northern Belize (Central America). Diversity and Distributions 10:189–199
Rejmánková E, Macek P, Epps K (2008) Wetland ecosystem changes after three years of phosphorus addition. Wetlands 28:914–927
Rejmánková E, Sirová D, Carlson E (2011) Patterns of activities of root phosphomonoesterase and phosphodiesterase in wetland plants as a function of macrophyte species and ambient phosphorus regime. The New Phytologist 190:968–976
Šantrůčková H, Rejmánková E, Pivničková B, Snyder JM (2010) Nutrient enrichment in tropical wetlands: shift from autotrophic to heterotrophic nitrogen fixation. Biogeochemistry 101:295–310
Sauer D, Kuzyakov Y, Stahr K (2006) Short communication spatial distribution of root exudates of five plant species as assessed by 14C labeling. Journal of Plant Nutrition and Soil Science 196:360–362
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Sillen WMA, Dieleman WIJ (2012) Effects of elevated CO2 and N fertilization on plant and soil carbon pools of managed grasslands: a meta-analysis. Biogeosciences 9:2247–2258
Sirová D, Vrba J, Rejmánková (2006) Extracellular enzyme activities in benthic cyanobacterial mats: comparison between nutrient enriched and control sites in marshes of northern Belize. Applied Microbial Ecology 44:11–20
Sirová D, Rejmánková E, Carlson E, Vrba J (2013) Current standard assays using artificial substrates overestimate phosphodiesterase activity. Soil Biology and Biochemistry 56:75–79
Ström L, Ekberg A, Mastepanov M, Christensen TR (2003) The effect of vascular plants on carbon turnover and methane emissions from a tundra wetlands. Global Change Biology 9:1185–1192
Uren NC (2001) Types, amounts and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil-plant Interface. Marcel Dekker, New York, pp. 19–40
Warembourg FR, Roumet C, Lafont F (2003) Differences in rhizosphere carbon-partitioning among plant species from different families. Plant and Soil 256:347–357
Weidie AE (1985) Geology of the Yucatan platform. In: Ward WC, Weidie AE, Back W (eds) Geology and hydrology of the Yucatan and quaternary geology of Northeastern Yucatan Peninsula. New Orleans Geological Society, New Orleans, pp. 1–19
Acknowledgments
We would like to thank to Irenio Briceno and Russel King for their assistance in the field and to Emily Carlson, Tereza Říhová and Daniel Vaněk for their laboratory assistance. Language correction by Stephanie Castle is greatly appreciated. This research was supported by the following grants: National Science Foundation ( NSF # 0089211) to E.R.; Ministry of Youth Sports and Education (ME 912 and LM2015075) and Grant Agency of the University of South Bohemia (GA JU 146/2013P) to H.Š.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šantrůčková, H., Kubešová, J., Šantrůček, J. et al. The Effect of P Enrichment on Exudate Quantity and Bioavailability - a Comparison of Two Macrophyte Species. Wetlands 36, 789–798 (2016). https://doi.org/10.1007/s13157-016-0785-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-016-0785-0