Abstract
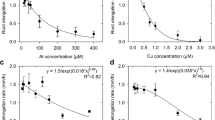
The uptake of trace metals by plants is commonly assumed to depend on the free metal-ion activity, rather than on the total concentration of dissolved metal. Although this free-ion hypothesis has proved to be useful for the interpretation and prediction of metal uptake, several exceptions have been reported where metal complexes also affected metal uptake by plants. In this study, we measured uptake of Zn and Cu by spinach (Spinacia oleracea L.) and tomato (Lycopersicon esculentum L.) in chelator-buffered or resin (Chelex)-buffered solutions, under Zn-deficient and non-deficient conditions. Several ligands, with differing dissociation rates, were used in the chelator-buffered solutions. At the same free-ion activity, Cu and Zn uptake was less in Chelex-buffered than in chelator-buffered solutions. In the chelator-buffered solution, uptake of Cu and Zn at same free-ion activity and same total concentration followed the order: NTA > HEDTA > EDTA > CDTA, i.e., the same order as the dissociation rate. These differences in metal uptake were also reflected in the deficiency symptoms and plant yield in the experiments where Zn deficiency was imposed. The critical Zn2+ activity for Zn deficiency varied by one order of magnitude depending on the buffer, and followed the order HEDTA < CDTA < resin-buffered (no soluble ligand). These results suggest that, when present, aqueous complexes can increase metal uptake by plants because uptake is rate-limited by diffusion of the free ion to the root or cell surface. Thus, the critical free-ion activity in chelator-buffered solutions depends on the type and concentration of the ligand employed.



Similar content being viewed by others
References
Bell PF, Chaney RL, Angle JS (1991) Free metal activity and total metal concentrations as indices of micronutrient availability to barley [Hordeum-vulgare (L.) ‘Klages’]. Plant Soil 130:51–62
Berkelaar EJ, Hale BA (2003) Cadmium accumulation by durum wheat roots in ligand-buffered hydroponic culture: uptake of Cd-ligand complexes or enhanced diffusion? Can J Bot 81:755–763
Buxani-Rice S, Ueda F, Bradbury MWB (1994) Transport of zinc−65 at the blood–brain barrier during short cerebrovascular perfusion in the rat: its enhancement by histidine. J Neurochem 62:665–672
Cakmak I, Marschner H (1988) Increase in membrane permeability and exudation in roots of zinc deficient plants. J Plant Physiol 132:356–361
Cakmak I, Sari N, Marschner H, Kalayci M, Yilmaz A, Eker S, Gülüt KY (1996) Dry matter production and distribution of zinc in bread and durum wheat genotypes differing in zinc efficiency. Plant Soil 180:173–181
Chaney RL (1988) Plants can utilize iron from Fe-N,N′-di-(2-Hydroxybenzoyl)-ethylenediamine-N,N′-diacetic acid, a ferric chelate with 106 greater formation constant than Fe-EDDHA. J Plant Nutr 11:1033–1050
Chaney RL, Bell PF, Coulombe BA (1989) Screening strategies for improved nutrient uptake and utilization by plants. HortSci 24:565–572
Checkai RT, Hendrickson LL, Corey RB, Helmke PA (1987) A method for controlling the activities of free metal, hydrogen and phosphate ions in hydroponic solutions using ion exchange and chelating resins. Plant Soil 99:321–334
Checkai RT, Norvell WA (1992) A recirculating resin-buffered hydroponic system for controlling nutrient ion activities. J Plant Nutr 15:871–892
Collins RN, Merrington G, McLaughlin MJ, Knudsen C (2002) Uptake of intact zinc-ethylenediaminetetraacetic acid from soil is dependent on plant species and complex concentration. Environ Toxicol Chem 21:1940–1945
Degryse F, Smolders E, Merckx R (2006) Labile Cd complexes increase Cd availability to plants. Environ Sci Technol 40:830–836
Hudson RJM (1998) Which aqueous species control the rates of trace metal uptake by aquatic biota? Observations and predictions of non-equilibrium effects. Sci Total Environ 219:95–115
Hudson RJM, Morel FMM (1993) Trace-metal transport by marine microorganisms—implications of metal coordination kinetics. Deep-Sea Res 40:129–150
Jansen S, Blust R, Van Leeuwen HP (2002) Metal speciation dynamics and bioavailability: Zn(II) and Cd(II) uptake by mussel (Mytilus edulis) and carp (Cyprinus carpio). Environ Sci Technol 36:2164–2170
Keller H, Römer W (2001) Cu, Zn, and Cd acquisition by two spinach cultivars depending on P nutrition and root exudation. J Plant Nutr Soil Sci 164:335–342
Lombnæs P, Singh BR (2003) Varietal tolerance to zinc deficiency in wheat and barley grown in chelator-buffered nutrient solution and its effect on uptake of Cu, Fe, and Mn. J Plant Nutr Soil Sci 166:76–83
Macnicol RD, Beckett PHT (1985) Critical tissue concentrations of potentially toxic elements. Plant Soil 85:107–129
McLaughlin MJ, Smolders E, Merckx R and Maes A (1997) Plant uptake of Cd and Zn in chelator-buffered nutrient solution depends on ligand type. In: Ando T et al (eds) Plant nutrition—for sustainable food production and environment. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 113–118
Meylan S, Behra R, Sigg L (2004) Influence of metal speciation in natural freshwater on bioaccumulation of copper and zinc in periphyton: a microcosm study. Environ Sci Technol 38:3104–3111
Nolan AL, McLaughlin MJ, Mason SD (2003) Chemical speciation of Zn, Cd, Cu and Pb in pore waters of agricultural and contaminated soils using donnan dialysis. Environ Sci Technol 37:90–98
Norvell WA, Welch RM (1993) Growth and nutrient uptake by barley (Hordeum vulgare L. cv Herta): Studies using an N-(2-hydroxyethyl)ethylenedinitrilotriacetic acid buffered nutrient solution technique. I. Zinc ion requirements. Plant Physiol 101:619–625
Parker DR (1993) Novel nutrient solutions for zinc nutrition research: buffering free zinc2+ with synthetic chelators and P with hydroxyapatite. Plant Soil 156:461–464
Parker DR (1997) Responses of six crop species to solution zinc2+ activities buffered with HEDTA. Soil Sci Soc Am J 61:167–176
Parker DR, Aguilera JJ, Thomason DN (1992) Zinc-phosphorus interactions in two cultivars of tomato (Lycopersicon esculentum L.) grown in chelator-buffered nutrient solutions. Plant Soil 143:163–177
Parker DR, Chaney RL and Norvell WA (1995a) Chemical equilibrium models: applications to plant nutrition research. In: Loeppert RH, Schwab AP, Goldberg S (eds) Chemical equilibrium and reaction models. Soil Sci. Soc. Amer. Spec. Pub. No. 42, Madison, WI, pp 163–200
Parker DR, Norvell WA and Chaney RL (1995b) GEOCHEM-PC: a chemical speciation program for IBM and compatible personal computers. In: Loeppert RH, Schwab AP, Goldberg S (eds) Chemical equilibrium and reaction models. Soil Sci. Soc. Amer. Spec. Pub. No. 42, Madison, WI, pp 253–269
Tiller KG, Honeysett JL, de Vries MPC (1972) Soil zinc and its uptake by plants. II. Soil chemistry in relation to prediction of availability. Aust J Soil Res 10:165–182
van Leeuwen HP (2001) Revisited: the conception of lability of metal complexes. Electroanal 13:826–830
Vassil AD, Kapulnik Y, Raskin I, Salt DE (1998) The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiol 117:447–453
Webb MJ, Norvell WA, Welch RM, Graham RD (1993) Using a chelate-buffered nutrient solution to establish the critical solution activity of Mn2+ required by barley (Hordeum vulgare L.). Plant Soil 153:195–205
Weisiger RA, Pond SM, Bass L (1989) Albumin enhances unidirectional fluxes of fatty acid across a lipid-water interface: theory and experiments. Am J Physiol 257:G904–G916
Wilkinson KJ, Buffle J (2004) Critical evaluation of physicochemical parameters and processes for modelling the biological uptake of trace metals in environmental (aquatic) systems. In: van Leeuwen HP, Koester W (eds) Physicochemical kinetics and transport at biointerfaces. John Wiley & Sons, Ltd., pp 445–533
Yang X, Römheld V, Marschner H, Chaney RL (1994) Application of chelator-buffered nutrient solution technique in studies on zinc nutrition in rice plant (Oryza sativa L.). Plant Soil 163:85–94
Zhang FS, Römheld V, Marschner H (1991) Release of zinc mobilizing root exudates in different plant-species as affected by zinc nutritional status. J Plant Nutr 14:657–686
Zhang H, Zhao F-J, Sun B, Davison W, McGrath SP (2001) A new method to measure effective soil solution concentration predicts copper availability to plants. Environ Sci Technol 35:2602–2607
Acknowledgement
F. Degryse thanks the Fund for Scientific Research—Flanders (FWO) for a postdoctoral fellowship and a travel grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Degryse, F., Smolders, E. & Parker, D.R. Metal complexes increase uptake of Zn and Cu by plants: implications for uptake and deficiency studies in chelator-buffered solutions. Plant Soil 289, 171–185 (2006). https://doi.org/10.1007/s11104-006-9121-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9121-4