Abstract
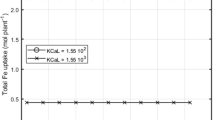
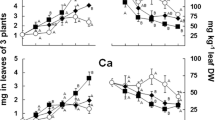
The form in which a micronutrient is found in the rhizosphere affects its availability to plants. We compared the availability to barley of the free hydrated cation form of Fe3+, Cu2+, Zn2+, and Mn2+ versus their total metal concentrations (free ion plus complexes) in chelator-buffered solutions. Free metal ion activities were estimated using the chemical equilibrium program GEOCHEM-PC with the corrected database. In experiment 1, barley was grown in nutrient solutions with different Fe3+ activities using chelators to control Fe levels. Chlorosis occurred at Fe3+ activities of 10−18 and 10−19 M for barley grown in HEDTA and EDTA solutions, respectively. In experiment 2, barley was grown in nutrient solutions with the same calculated Fe3+ activity and the same chelator, but different total Fe concentrations. Leaf, root and shoot Fe concentrations were higher from CDTA buffered solutions which had the higher total Fe concentration indicating the importance of the total Fe concentration on Fe uptake. Results from treatments using EDTA or HEDTA, with one exception, were similar to the results from the CDTA treatment. This suggests differences in critical Fe3+ activities found in experiment 1 were due to differences in the total Fe concentration and not errors in chelate formation constants used to estimate the critical activities. Results for Cu, Zn, and Mn were similar to Fe; despite solutions with equal free Cu2+, Zn2+ and Mn2+ activities, plant concentrations of these metals were generally greater when grown in the solutions with the greater total amount of Cu, Zn, or Mn. When the free Zn2+ activity was kept constant while the total amount of Zn was increased from 4.4 to 49 μM, leaf Zn concentration increased from 77 to 146 μg g-1. In order to predict metal availability to barley and other species in chelator-buffered nutrient solutions, both free and total metal concentrations in solution must be considered. The critical Fe3+ activities required by barley in this study are much higher than those from tomato and soybean, 10-28 M, which strongly supports the Strategy 2 model of Fe uptake for Poaceae. This is related to the importance of the Fe3+ (barley) and the Fe2+ (tomato and soybean) ions in Fe uptake. Fe-stressed barley is known to release phytosiderophores which compete for Fe3+ in the nutrient solution, while tomato and soybean reduce Fe3+ to Fe2+ at the epidermal cell membranes to allow uptake of Fe2+ from Fe3+ chelates in solution.
Similar content being viewed by others
Abbreviations
- CDTA:
-
trans-1,2-diaminocyclohexane-N,N,N′,N′-tetracetic acid
- DTPA:
-
diethylenetriaminepentacetic acid
- EDTA:
-
ethylenediaminetetracetic acid
- EDDHA:
-
ethylenediamine-di(o-hydroxyphenylacetic acid)
- HBED-N,N′:
-
di(2-hydroxybenzoyl)-ethylenediamine-N,N′-diacetic acid
- HEDTA-N:
-
hydroxyethylenediaminetriacetic acid
- MES-2:
-
(N-morpholino)ethanesulfonic acid
- NTA:
-
nitrilotriacetic acid
References
Anderson D M and Morel F M M 1978 Copper sensitivity of Gonyaulax tamarensis. Limn. Ocean. 23, 282–295.
Awad F, Römheld V and Marschner H 1988 Mobilization of ferric iron from a calcareous soil by plant-borne chelators (phytosiderophores). J. Plant Nutr. 11, 701–713.
Bradford G R, Bair F L and Hunsaker V 1971 Trace and major element contents of soil saturation extracts. Soil Sci. 112, 225–230.
Brown J C, Tiffin L O and Holmes R S 1960 Competition between chelating agents and roots as factor affecting absorption of iron and other ions by plant species. Plant Physiol. 35, 878–886.
Bugbee B G 1987 Maintenance of recirculating hydroponic systems. Hortscience 22, 1000.
Chaney R L 1988a Plants can utilize iron from Fe-N,N′-di-(2-hydroxybenzoyl)-ethylenediamine-N-N′-diacetic acid, a ferric chelate with 106 greater formation constant than Fe-EDDHA. J. Plant Nutr. 11, 1033–1050.
Chaney R L 1988b Metal speciation and interactions among elements affect trace element transfer in agricultural and environmental food-chains. In Metal Speciation: Theory, Analysis and Application. Eds. J RKramer and H E Allen. pp 219–260. Lewis Publishers, Chelsea, MI.
Chaney R L, Brown J C and Tiffin L O 1971 Obligator reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 50, 208–213.
Chaney R L, Bell P F and Melo I F S 1988 Activity of microelement cations required by plants in DTPA-buffered nutrient solutions. Agron. Abstr. 1988, 231.
Chaney R L, Bell P F and Coulombe B A 1989 Screening strategies for improved nutrient uptake and utilization by plants. HortScience 24, 565–572.
Checkai R T, Corey R B and Helmke P A 1987 Effects of ionic and complexed metal concentrations on plant uptake of cadmium and micronutrient metals from solution. Plant and Soil 99, 335–345.
DeKock P C and Mirchell R L 1957 Uptake of chelated metals. Soil Sci. 84, 55–62.
Halvorson A D and Lindsay W L 1977 The critical Zn2+ concentration for corn and the nonabsorption of chelated zinc. Soil Sci. Soc. Am. J. 41, 531–534.
Häussling M, Jorns C A, Lehmbecker G, Hecht-Buchholz Ch and Marschner H 1988 Ion and water uptake in relation to root development in Norway spruce (Picea abies (L.) Karst.). J. Plant Physiol. 133, 486–491.
Jeffreys R A and Wallace A 1968 Detection of iron ethylenediamine di(o-hydroxyphenylacetate) in plant tissues. Agron. J. 60, 613–616.
Kramer P J 1969 Plant and Soil Water Relationships. McGraw-Hill, New York.
L'Eplattenier, Murase F I and Martell A E 1967 New multidentate ligands. VI. Chelating tendencies of N,N′-di(2-hydroxybenzoyl)ethylene-diamine-N-N′-diacetic acid. J. Am. Chem. Soc. 89, 837–843.
Margerum D W, Cayley G R, Weatherburn D C and Pagenkopf G K 1978 Kinetics and mechanisms of complex formation and ligand exchange. In Coordination Chemistry, Volume 2. Ed. A E Martell. pp 1–220. ACS monog. 174, Washington, DC.
Marschner H, Treeby M and Römheld V 1989 Role of root-induced changes in the rhizosphere for iron acquisition in higher plants. Z. Pflanzenernachr. Bodenkd. 152, 197–204.
McGrath S P, Sanders J R, Laurie S H and Tancock N P 1986 Experimental determinations and computer predictions of trace metal ion concentrations in dilute complex solutions. Analyst 111, 459–465.
Parker D R, Zelazny L W and Kinraide T B 1987 Improvements to the program GEOCHEM Soil Sci. Soc. Am. J. 51, 488–491.
Peterson C A, Emanuel M E and Humphreys G B 1981 Pathway of movement of apoplastic fluorescent dye tracers through the endodermis at the site of secondary root formation in corn (Zea mays) and broad bean (Vidia faba). Can J. Bot. 59, 618–625.
Rasmussen H P 1968 Entry and distribution of aluminum in Zea mays. Planta (Berlin) 81, 28–37.
Rogers H J 1973 Iron-binding catechols and virulence in Escherichia coli. Infect. Immunity 7, 445–456.
Römheld V and Marschner H 1981a Effect of Fe stress on utilization of Fe chelates by efficient and inefficient plant species. J. Plant Nutr. 3, 1–4.
Römheld V and Marschner H 1981b Rhythmic iron stress reactions in sunflower at suboptimal iron supply. Physiol. Plant. 53, 347–353.
Römheld V and Marschner H 1986 Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 80, 175–180.
SAS Institute Inc 1986 SUGI Supplemental Library User's Guide, Version 5 Edition. SAS Institute, Cary, NC, 662 p.
Sposito G and Mattigod S V 1980 GEOCHEM: A computer program for the calculation of chemical equilibria in soil solutions and other natural water systems. Kearney Foundation of Soil Science, University of California, Riverside, 92 p.
Sugiura Y, Tanaka H, Mino Y, Ishida I, Ota N, Inoue M, Nomoto K, Yoshioka H and Takemoto T 1981 Structure of 2′-deoxymugineic acid, a novel amino acid possessing an iron-chelating activity. Chimia 35, 249–250.
Sunda W G and Guillard R R 1976 Relationship between cupric ion activity and the toxicity of copper to phytoplankton. J. Mar. Res. 34, 511–529.
Takagi S-I, Nomoto K and Takemoto T 1984 Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 7, 469–477.
Taylor G J and Foy C D 1985 Differential uptake and toxicity of ionic and chelated copper in Triticum aestivum. Can. J. Bot. 63, 1271–1275.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bell, P.F., Chaney, R.L. & Angle, J.S. Free metal activity and total metal concentrations as indices of micronutrient availability to barley [Hordeum vulgare (L.) ‘Klages’]. Plant Soil 130, 51–62 (1991). https://doi.org/10.1007/BF00011855
Issue Date:
DOI: https://doi.org/10.1007/BF00011855