Abstract
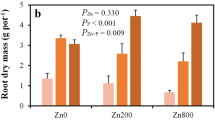
Chelator-buffered nutrient solutions, in which computed free Zn2+ activities are buffered at ≤10-10.0 M by including an excess of a synthetic chelator such as EDTA, have recently shown promise as a means of precisely regulating Zn nutritional status. A further refinement that would eliminate the confounding effect of high (and often phytotoxic) shoot P concentrations in solution-grown, Zn-deficient plants is also desirable. Several crop species were grown in 120-L of HEDTA-buffered solutions that contained just 10+-1 μM P. Critical free Zn2+ activities ranged from ≈10 to 60 pM, and relative yields as low as 32% of control were achieved. Concentrations of P in the older leaves were very high (up to 46 mg g-1) at low (Zn2+), suggesting that P toxicity can occur even without the high P concentrations (about 1 mM) typically used in Hoagland-type solutions. A second study was undertaken to better simulate soil conditions, wherein diffusion of P from the solid phase to the root is rate-limiting. Commercial hydroxyapatite (HAP) was enclosed in a pouch constructed of dialysis tubing, such that dissolution and diffusion occurred in response to plant depletion of P. Maize (Zea mays L.) and wheat (Triticum aestivum L.) can be supplied with P at adequate levels using this approach, and acutely Zn-deficient plants did not hyperaccumulate P. However, two dicots tested were too P-inefficient to grow normally with HAP as the sole P source.
Similar content being viewed by others
References
Barber S A 1984 Soil Nutrient Bioavailability. Johns Wiley and Sons, New York. 398 p.
Bell P F, Chaney R L and Angle J S 1991 Soil Sci. Soc. Am. J. 55, 1366–1374.
Chaney R L, Bell P F and Coulombe B A 1980 HortScience 24, 565–572.
Lindsay W L 1979 Chemical Equilibria in Soils. John Wiley and Sons, New York. 449 p.
Loneragan J F, Grove T S, Robson A D and Snowball K 1979 Soil Sci. Soc. Am. J. 43, 966–972.
Loneragan J F, Grunes D L, Welch R M, Aduayi E A, Tengh A, Lazar V A and Cary E E 1982 Soil Sci. Soc. Am. J. 46, 345–352.
Marschner H and Cakmak I 1986 Physiol. Plant. 68, 491–496.
Martell A E and Smith R M 1976–1989 Critical Stability Constants. 6 Volumes. Plenum Press, New York.
Norvell W A 1991 In Micronutrients in Agriculture. 2nd edition. Ed. J. J. Mordvedt. pp. 187–227. SSSA Book Ser. No. 4. Soil Sci. Soc. Amer., Madsion, WI.
Norvell W A and Welch R M 1993 Plant Physiol. 101, 619–625.
Parker D R, Aguilera J J and Thomason D N 1992 Plant Soil 143, 163–177.
Parker D R, Chaney R L and Norvell W A 1993a In Soil Chemical Equilibrium and Reaction Models. Ed. R H Loeppert. Soil Sci Soc. Amer., Madison, WI. (in press).
Parker D R, Norvell W A and Chaney R L 1993b In Soil Chemical Equilibrium and Reaction Models. Ed. R H Loeppert. Soil Sci Soc. Amer., Madison, WI. (in press).
Webb M J and Loneragan J F 1988 Soil Sci. Soc. Am. J. 52.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parker, D.R. Novel nutrient solutions for zinc nutrition research: buffering free zinc2+ with synthetic chelators and P with hydroxyapatite. Plant Soil 155, 461–464 (1993). https://doi.org/10.1007/BF00025083
Issue Date:
DOI: https://doi.org/10.1007/BF00025083