Abstract
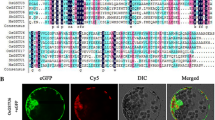
Glutathione reductases (GRs) are important components of the antioxidant machinery that plants use to respond against abiotic stresses. In rice, one cytosolic and two chloroplastic GR isoforms have been identified. In this work, we describe the cloning and characterization of the full-length cDNA encoding OsGR3, a chloroplast-localized GR that up to now was considered as a non-functional enzyme because of assumed lack of N-terminal conserved domains. The expression of OsGR3 in E. coli validated that it can be translated as a protein with GR activity. OsGR3 shows 76 and 53 % identity with OsGR1 (chloroplastic) and OsGR2 (cytosolic), respectively. Phylogenetic analysis revealed 2 chloroplastic GRs in Poaceae species, including rice, sorghum and brachypodium, but only one chloroplastic GR in dicots. A plastid transit peptide is located at the N terminus of OsGR3, and genetic transformation of rice with a GR3-GFP fusion construct further confirmed its localization in chloroplasts. Furthermore, OsGR1 and OsGR3 are also targeted to mitochondria, which suggest a combined antioxidant mechanism in both chloroplasts and mitochondria. However, both isoforms showed a distinct response to salinity: the expression of OsGR3 but not OsGR1 was induced by salt stress. In addition, the transcript level of OsGR3 was greatly increased with salicylic acid treatment but was not significantly affected by methyl jasmonate, dehydration or heat shock stress. Our results provide new clues about the possible roles of functional OsGR3 in salt stress and biotic stress tolerance.








Similar content being viewed by others
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Azevedo-Neto AD, Pitsco JT, Eneas-Filho J, de Abreu CEB, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Bashir K, Nagasaka S, Itai RN, Kobaysahi T, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2007) Expression and enzyme activity of glutathione reductase is upregulated by Fe-deficiency in graminaceous plants. Plant Mol Biol 64:277–284
Benke D, Cicin-Sain A, Mertens S, Mohler H (1991) Immunochemical identification of the α1- and α3-subunits of the GABAA-receptor in rat brain. J Rec Res 11:407–424
Chen YP, Xing LP, Wu GJ, Wang HZ, Wang XE, Cao AZ, Chen PD (2007) Plastidial glutathione reductase from Haynaldia villosa is an enhancer of powdery mildew resistance in wheat (Triticum aestivum). Plant Cell Physiol 48:1702–1712
Chew O, Whelan J, Millar AH (2003) Molecular definition of the ascorbate–glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J Biol Chem 278:46869–46877
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgen Res 5:213–218
Comba ME, Benavides MP, Tomaro ML (1998) Effect of salt stress on antioxidant defense system in soybean root nodules. Aus J Plant Physiol 25:665–671
Contour-Ansel D, Torres-Franklin LM, de Carvalho MHC, D’Arcy-Lameta A, Zuily-Fodil Y (2006) Glutathione reductase in leaves of cowpea: cloning of two cDNAs, expression and enzymatic activity under progressive drought stress, desiccation and abscisic acid treatment. Ann Bot 98:1279–1287
Creissen GP, Mullineaux PM (1995) Cloning and characterization of glutathione reductase cDNAs and identification of two genes encoding the tobacco enzyme. Planta 197:422–425
Creissen GP, Edwards EA, Enard C, Wellburn A, Mullineaux PM (1991) Molecular characterization of glutathione reductase cDNAs from pea (Pisum sativum L.). Plant J 2:129–131
Dinakar C, Abhaypratap V, Yearla SR, Raghavendra AS, Padmasree K (2010) Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta 231:461–474
Ding S, Lu Q, Zhang Y, Yang Z, Wen X, Zhang L, Lu C (2009) Enhanced sensitivity to oxidative stress in transgenic tobacco plants with decreased glutathione reductase activity leads to a decrease in ascorbate pool and ascorbate redox state. Plant Mol Biol 69:577–592
Ding S, Lei M, Lu Q, Zhang A, Yin Y, Wen X, Zhang L, Lu C (2012) Enhanced sensitivity and characterization of photosystem II in transgenic tobacco plants with decreased chloroplast glutathione reductase under chilling stress. Biochim Biophys Acta 1817:1979–1991
Dionisio-Sese ML, Tobita S (2007) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Edwards EA, Rawsthorne S, Mullineaux PM (1990) Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 180:278–284
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300:1005–1016
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signaling. New Phytol 146:359–388
Ganesan V, Thomas G (2001) Salicylic acid response in rice: influence of salicylic acid on H2O2 accumulation and oxidative stress. Plant Sci 160:1095–1106
Garretón V, Carpinelli J, Jordana X, Holuigue L (2002) The as-1 promoter element is an oxidative stress-responsive element and salicylic acid activates it via oxidative species. Plant Physiol 130:1516–1526
Hernandez JA, Francisco J, Corpas FJ, del Rio LA, Sevilla F (1993) Salt induced oxidative stresses mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant 89:103–110
Hernandez JA, Jimenez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long term salt stress is associated with induction of antioxidant defences. Plant, Cell Environ 23:853–862
Hong CY, Cheng KJ, Tseng TH, Wang CS, Liu LF, Yu SM (2004) Production of two highly active bacterial phytases with broad pH optima in germinated transgenic rice seeds. Transgen Res 13:29–33
Hong CY, Chao YY, Yang MY, Cheng SY, Cho SC, Kao CH (2009a) NaCl-induced expression of glutathione reductase in roots of rice (Oryza sativa L.) seedlings is mediated through hydrogen peroxide but not abscisic acid. Plant Soil 320:103–115
Hong CY, Chao YY, Yang MY, Cho SC, Kao CH (2009b) Na+ but not Cl− or osmotic stress is involved in NaCl-induced expression of glutathione reductase in roots of rice seedlings. J Plant Physiol 166:1598–1606
Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinform 2008:420747
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049
Jimenez A, Hernandez J, del Rio L, Sevilla F (1997) Evidence for the presence of the ascorbate–glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Kaminaka H, Morita S, Nakajima M, Masumura T, Tanaka K (1998) Gene cloning and expression of cytosolic glutathione reductase in rice (Oryza sativa L.). Plant Cell Physiol 39:1269–1280
Kubo A, Sano T, Saji H, Tanaka K, Kondo N, Tanaka K (1993) Primary structure and properties of glutathione reductase from Arabidopsis thaliana. Plant Cell Physiol 34:1259–1266
Laloi C, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld J-P (2004) The arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-Box-mediated response to pathogen elicitor. Plant Physiol 134:1006–1016
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Le Martret B, Poage M, Shiel K, Nugent GD, Dix PJ (2011) Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol J 9:661–673
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) The reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mittova V, Theodoulou FL, Kiddle G, Gomez L, Volokita M, Tal M, Foyer CH, Guy M (2003) Coordinate induction of glutathione biosynthesis and glutathione-metabolizing enzymes is correlated with salt tolerance in tomato. FEBS Lett 554:417–421
Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid- plant soil mediated oxidative damage requires H2O2. Plant Physiol 115:137–149
Rouhier N, Couturier J, Jacquot JP (2006) Genome-wide analysis of plant glutaredoxin systems. J Exp Bot 57:1685–1696
Sairam RK, Srivastava GC, Agarwal S, Meena RC (2005) Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–91
Scandalios JG (2002) The rise of ROS. Trends Biochem Sci 27:483–486
Sekmen AH, Türkan I, Takio S (2007) Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol Plant 131:399–411
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
Shu DF, Wang LY, Duan M, Deng YS, Meng QW (2011) Antisense-mediated depletion of tomato chloroplast glutathione reductase enhances susceptibility to chilling stress. Plant Physiol Biochem 49:1228–1237
Stevens RG, Creissen GP, Mullineaux PM (1997) Cloning and characterisation of a cytosolic glutathione reductase cDNA from pea (Pisum sativum L.) and its expression in response to stress. Plant Mol Biol 35:641–654
Sun H, Li L, Wang X, Wu S, Wang X (2010) Ascorbate–glutathione cycle of mitochondria in osmoprimed soybean cotyledons in response to imbibitional chilling injury. J Plant Physiol 168:226–232
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Torres-Franklin ML, Contour-Ansel D, Zuily-Fodil Y, Pham-Thi AT (2008) Molecular cloning of glutathione reductase cDNAs and analysis of GR gene expression in cowpea and common bean leaves during recovery form moderate drought stress. J Plant Physiol 165:514–521
Tsai YC, Hong CY, Liu LF, Kao CH (2005) Expression of ascorbate peroxidase and glutathione reductase in roots of rice seedlings in response to NaCl and H2O2. J Plant Physiol 162:291–299
Xu L, Carrie C, Law SR, Murcha MW, Whelan J (2013) Acquisition, conservation, and loss of dual-targeted proteins in land plant. Plant Physiol 161:644–662
Yoshida S, Forno DA, Cock JH, Gomez KA (1972) Laboratory manual for physiological studies of rice, 2nd edn. The International Rice Research Institute, Los Baños
Zhang Y, Su J, Duan S et al (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7:30–43
Acknowledgments
We thank Laura Smales for English editing. We are grateful to the Joint Center for Instruments and Researches of the College of Bioresources and Agriculture at National Taiwan University for confocal microscopy and technical support. This work was supported by research grants from the National Science Council of the Republic of China (NSC 98-2324-B-002-002 and NSC 98-2313-B-002-015-MY3) to C.-Y. Hong.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2013_95_MOESM1_ESM.ppt
Fig S1. Alignment of the 5’ RACE product with truncated cDNA and the final assembled OsGR3 gene. DNA sequence alignment for OsGR3 5’RACE product, truncated OsGR3 cDNA (Tc-OsGR3; accession no. AK108799) from databases and final assembled gene (this study). Identical residues with the final assembled gene (GR3 cDNA) are on a gray background. DNA sequence overlap between the 5’ RACE product, Tc-OsGR3 and the final assembled gene are on a black background. The alignment was generated by use of Clustal W 2.0 (Larkin et al., 2007) (PPT 824 kb)
11103_2013_95_MOESM2_ESM.ppt
Fig S2. Alignment of the OsGR3 complete coding sequence with rice genome sequences. Identical residues with exon sequences are in white on a black background. Identical residues with intron sequences are in black on a gray background. The alignment was generated by use of Clustal W 2.0 (Larkin et al., 2007) (PPT 1799 kb) (PPT 1099 kb)
Rights and permissions
About this article
Cite this article
Wu, TM., Lin, WR., Kao, YT. et al. Identification and characterization of a novel chloroplast/mitochondria co-localized glutathione reductase 3 involved in salt stress response in rice. Plant Mol Biol 83, 379–390 (2013). https://doi.org/10.1007/s11103-013-0095-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-013-0095-3