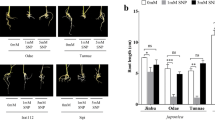
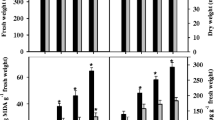
Abstract
Reactive oxygen species (ROS) play an important role in NaCl stress. Plants tolerant to NaCl stress may evolve certain strategies to remove these ROS, thus reducing their toxic effects. Therefore, the expression patterns of the gene family encoding glutathione reductase (GR, EC 1.6.4.2) were analyzed in roots of etiolated rice (Oryza sativa L.) seedlings in response to NaCl stress. Semi-quantitative RT-PCR was applied to quantify the mRNA levels for one cytosolic (OsGR2) and two chloroplastic (OsGR1 and OsGR3) isoforms of glutathione reductase identified in the rice genome. The expression of OsGR2 and OsGR3 but not OsGR1 was increased in rice roots treated with 150 mM NaCl. The Rab16A is an abscisic acid (ABA)-responsive rice gene. Increasing concentrations of ABA, from 1 to 12 μM, progressively increased the expression of OsRab16A in rice roots. In the present study, the ABA level was judged by the expression of OsRab16A in rice roots. Treatment with 150 mM NaCl induced the expression of OsRab16A, and the expression increased with increasing concentrations of ABA, which suggests that ABA may be involved in this response in rice roots. In fact, exogenous application of ABA enhanced the expression of OsGR2 and OsGR3 in rice roots. On inhibiting ABA accumulation with sodium tungstate (Tu), an inhibitor of ABA biosynthesis, the expression of OsGR2 and OsGR3 was still induced by NaCl; therefore, NaCl-triggered expression of OsGR2 and OsGR3 in rice roots is not mediated by accumulation of ABA. However, NaCl treatment could induce H2O2 production in rice roots, and H2O2 treatment resulted in enhanced OsGR2 and OsGR3 induction. On inhibiting the NaCl-induced accumulation of H2O2 with diphenylene iodonium, the expression of OsGR2 and OsGR3 was also suppressed. Moreover, the increase in H2O2 level was prior to the induction of OsGR2 and OsGR3 in NaCl-treated rice roots. Thus, H2O2, but not ABA, is involved in regulation of OsGR2 and OsGR3 expression in NaCl-treated rice roots.










Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- Asc:
-
Ascorbic acid
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DCF-DA:
-
2′, 7′-Dichlorofluorescein diacetate
- DPI:
-
Diphenylene iodonium
- DW:
-
Dry weight
- ELISA:
-
Enzyme-linked immunosorbent assay
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- Tu:
-
Sodium tungstate
References
Alscher RG (1989) Biosynthesis and anti-oxidant function of glutathione in higher plants. Physiol Plant 77:457–464 doi:10.1111/j.1399-3054.1989.tb05667.x
Aono M, Kubo A, Saji H, Natori T, Tanaka K, Kondo N (1991) Resistance of active oxygen toxicity of transgenic Nicotiana tabacum that expresses the gene for glutathione reductase from Escherichia coli. Plant Cell Physiol 32:691–697
Aono M, Kubo A, Saji H, Tanaka K, Kondo N (1993) Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol 34:129–135
Aydi S, Gassi S, Abdelly C (2008) Growth, nitrogen fixation and ion distribution in Medicago truncatula subjected to salt stress. Plant Soil 312:59–67 doi:10.1007/s11104-008-9656-7
Bashir K, Nagasaka S, Itai RN, Kobaysahi T, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2007) Expression and enzyme activity of glutathione reductase is upregulated by Fe-deficiency in graminaceous plants. Plant Mol Biol 64:277–284 doi:10.1007/s11103-007-9216-1
Broadbent P, Creissen GP, Kular B, Wellburn AR, Mullineaux PM (1995) Oxidative stress responses in transgenic tobacco containing altered levels of glutathione reductase activity. Plant J 8:247–255 doi:10.1046/j.1365-313X.1995.08020247.x
Chang HS Chen W, Cooper B, Glazebrook J, Goff SA, Hou YM, Katagiri F, Quan S, Tao Y, Whitham S, Xie Z, Zhu T, Zou G (2003) Plant genes involved in defense against pathogens. European Patent EP1402037
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832 doi:10.1104/pp.123.3.825
Contour-Ansel D, Torres-Franklin LM, De Carvalho MHC, D’arcy-Lameta A, Zuily-Fodil Y (2006) Glutathione reductase in leaves of cowpea: cloning of two cDNAs, expression and enzymatic activity under progressive drought stress, desiccation and abscisic acid treatment. Ann Bot (Lond) 98:1279–1287 doi:10.1093/aob/mcl217
Cross AR (1990) Inhibitions of the leukocyte superoxide generating oxidase: mechanism of action and methods for their elucidation. Free Radic Biol Med 8:71–93 doi:10.1016/0891-5849(90)90147-B
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795 doi:10.1007/s000180050041
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9 doi:10.1016/S0168-9452(98)00025-9
Foster JG, Hess JL (1980) Superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487 doi:10.1104/pp.66.3.482
Foyer CH, Souriau N, Perret S, Lelandais M, Kunert K-J, Pruvost C, Jouanin L (1995) Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol 109:1047–1057 doi:10.1104/pp.109.3.1047
Frahry G, Schopfer P (1998) Inhibition of O2 - reducing activity of horseradish peroxidase by diphenyleneiodonium. Phytochemistry 48:223–227 doi:10.1016/S0031-9422(98)00004-1
Halliwell B, Gutteridge MJC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, London
Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124:1437–1408 doi:10.1104/pp.124.3.1437
Hernández JA, Almansa MS (2002) Short term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257 doi:10.1034/j.1399-3054.2002.1150211.x
Hernández JA, Ferrer MA, Jiméz A, Barceló AR, Sevilla F (2001) Antioxidant systems and O2.-/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol 127:817–831 doi:10.1104/pp.010188
Herouart D, van Montagu M, Inzè D (1993) Redox-activated expression of cytosolic copper/zinc superoxide dismutase gene in Nicotiana. Proc Natl Acad Sci U S A 90:3108–3112
Hong CY, Hsu YT, Tsai YC, Kao CH (2007) Expression of ASCORBATE PEROXIDASE 8 in roots of rice (Oryza sativa L.) seedlings in response to NaCl. J Exp Bot 58:3273–3283 doi:10.1093/jxb/erm174
Hung KT, Kao CH (2004) Hydrogen peroxide is necessary for abscisic acid senescence of rice leaves. J Plant Physiol 161:1347–1357 doi:10.1016/j.jplph.2004.05.011
Kaminaka H, Morita S, Nakajima M, Masumura T, Tanaka K (1998) Gene cloning and expression of cytosolic glutathione reductase in rice (Oryza sativa L.). Plant Cell Physiol 39:1269–1280
Kubo A, Sano T, Saji H, Tanaka K, Kondo N, Tanaka K (1993) Primary structure and properties of glutathione reductase from Arabidopsis thaliana. Plant Cell Physiol 34:1259–1266
Lee DH, Kim YS, Lee CB (2001) The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J Plant Physiol 158:737–745 doi:10.1078/0176-1617-00174
Levin A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593 doi:10.1016/0092-8674(94)90544-4
Lin CC, Kao CH (2001a) Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant Soil 230:135–143 doi:10.1023/A:1004876712476
Lin CC, Kao CH (2001b) Relative importance of Na+, Cl-, and abscsic acid in NaCl induced inhibition of root growth of rice seedlings. Plant Soil 237:165–171 doi:10.1023/A:1013321813454
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760 doi:10.1146/annurev.bi.52.070183.003431
Meot-Duros L, Magné C (2008) Effect of salinity and chemical factors on seed germination in the halophyte Crithmum maritimum L. Plant Soil 313:83–87 doi:10.1007/s11104-008-9681-6
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species, Lycopersicom pennellii. J Exp Bot 55:1105–1113 doi:10.1093/jxb/erh113
Montero E, Cabot C, Barceló J, Poschenrieder C (1997) Endogenous abscisic acid levels are linked to decreased growth of bush bean plants treated with NaCl. Physiol Plant 101:17–22 doi:10.1111/j.1399-3054.1997.tb01814.x
Moons A, Bauw G, Prinsen E, Van Montagu M, Van Der Straeten D (1995) Molecular and physiological responses to abscisic acid and salts in roots of salt-sensitive and salt-tolerant indica rice varieties. Plant Physiol 107:177–186 doi:10.1104/pp.107.1.177
Mundy J, Chua N-H (1988) Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J 7:2279–2286
Mundy J, Yamaguchi-Shinozaki K, Chua N-H (1990) Nucleaer proteins bind conserved elements in the abscisic acid-responsive promoter of rice rab gene. Proc Natl Acad Sci U S A 87:1406–1410 doi:10.1073/pnas.87.4.1406
Munns R (1993) Physiological responses limiting plant growth in saline soils: some dogma and hypothesis. Plant Cell Environ 16:1524
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signaling. Curr Opin Plant Biol 5:388–395 doi:10.1016/S1369-5266(02)00282-0
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279 doi:10.1146/annurev.arplant.49.1.249
Ogawa K, Tasaka Y, Mino M, Tanaka Y, Iwabuchi M (2001) Association of glutathione with flowering in Arabidopsis thaliana. Plant Cell Physiol 42:524–530 doi:10.1093/pcp/pce065
Orozco-Cárdenas ML, Narvaez-Váaquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13:179–191
Pei X, Murata Y, Benning G, thomine S, Klusener B, Allen G, Grill E, Schroeder J (2000) Calcium channel activated hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406:731–734 doi:10.1038/35021067
Sandalio LM, Rodríguez-Serrano M, Romero-Puertas MC, del Río LA (2008) Imaging of reactive oxygen species and nitric oxide in vivo in plant tissues. Methods Enzymol 440:397–409 doi:10.1016/S0076-6879(07)00825-7
Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2:503–512
Steffens JC (1990) The heavy metal-binding peptides of plants. Annu Rev Plant Physiol Plant Mol Biol 41:553–575
Stuehr PJ, Vasehun OH, Kwon NS, Grosss SS, Gonzalez JA, Levi R, Nathan CF (1991) Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analog. FASEB J 5:98–103
Sudhakar C, Lakshimi A, Gridarakumar S (2001) Changes in the antioxidant enzymes efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619 doi:10.1016/S0168-9452(01)00450-2
Timmerman KP (1989) Molecular characterization of corn glutathione S-transferase isozymes involved in herbicide detoxification. Physiol Plant 77:465–471 doi:10.1111/j.1399-3054.1989.tb05668.x
Torres-Franklin ML, Cotour-Ansel D, Zuily-Fodil Y, Pham-Thi A-T (2008) Molecular cloning of glutathione reductase cDNAs and analysis of GR gene expression in cowpea and common bean leaves during recovery form moderate drought stress. J Plant Physiol 165:514–521 doi:10.1016/j.jplph.2007.03.011
Tsai Y-C, Hong C-Y, Liu L-F, Kao CH (2004) Relative importance of Na+ and Cl- in NaCl-induced antioxidant systems in roots of rice seedlings. Physiol Plant 122:86–94 doi:10.1111/j.1399-3054.2004.00387.x
Tsai Y-C, Hong C-Y, Liu L-F, Kao CH (2005) Expression of ascorbate peroxidase and glutathione reductase in roots of rice seedlings in response to NaCl and H2O2. J Plant Physiol 162:291–299 doi:10.1016/j.jplph.2004.06.004
Vernoux T, Wilson RC, Seeley KA, Richheld J, Muroy S, Brown S, Maughan SC, Cobett CS, Van Montagu M, Inzè D, May MJ, Sung ZR (2000) The ROOT MERISTEMLESS/CADMIUM SENSITIVE 2 gene defines a glutathione dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12:97–110
Wingate VPM, Lawton MA, Lamb CJ (1983) Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol 87:206–210 doi:10.1104/pp.87.1.206
Wingsle G, Karpinski S (1995) Differential redox regulation by glutathione of glutathione reductase and CuZn-superoxide dismutase gene expression in Pinus sylvestris L. needles. Planta 198:151–157
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately response to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C-P (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448 doi:10.1104/pp.126.4.1438
Acknowledgements
We thank Dr. Nai-Chun Lin for critically reading the manuscript. This work was supported financially by the National Science Council of the Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John McPherson Cheeseman.
Rights and permissions
About this article
Cite this article
Hong, CY., Chao, YY., Yang, MY. et al. NaCl-induced expression of glutathione reductase in roots of rice (Oryza sativa L.) seedlings is mediated through hydrogen peroxide but not abscisic acid. Plant Soil 320, 103–115 (2009). https://doi.org/10.1007/s11104-008-9874-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9874-z