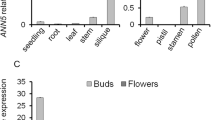
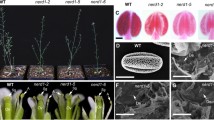
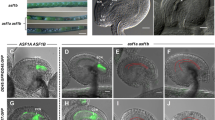
Abstract
Sexual plant reproduction depends on the production and differentiation of functional gametes by the haploid gametophyte generation. Currently, we have a limited understanding of the regulatory mechanisms that have evolved to specify the gametophytic developmental programs. To unravel such mechanisms, it is necessary to identify transcription factors (TF) that are part of such haploid regulatory networks. Here we focus on bZIP TFs that have critical roles in plants, animals and other kingdoms. We report the functional characterization of Arabidopsis thaliana AtbZIP34 that is expressed in both gametophytic and surrounding sporophytic tissues during flower development. T-DNA insertion mutants in AtbZIP34 show pollen morphological defects that result in reduced pollen germination efficiency and slower pollen tube growth both in vitro and in vivo. Light and fluorescence microscopy revealed misshapen and misplaced nuclei with large lipid inclusions in the cytoplasm of atbzip34 pollen. Scanning and transmission electron microscopy revealed defects in exine shape and micropatterning and a reduced endomembrane system. Several lines of evidence, including the AtbZIP34 expression pattern and the phenotypic defects observed, suggest a complex role in male reproductive development that involves a sporophytic role in exine patterning, and a sporophytic and/or gametophytic mode of action of AtbZIP34 in several metabolic pathways, namely regulation of lipid metabolism and/or cellular transport.








Similar content being viewed by others
References
Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira AP (1997) The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J 12:615–623. doi:10.1046/j.1365-313X.1997.00615.x
Alder NN, Shen Y, Brodsky JL, Hendershot LM, Johnson AE (2005) The molecular mechanisms underlying BiP-mediated gating of the Sec61 translocon of the endoplasmic reticulum. J Cell Biol 168:389–399. doi:10.1083/jcb.200409174
Allen RS, Li J, Stahle MI, Dubroue A, Gubler F, Millar AA (2007) Genetic analysis reveals functional redundancy and the major target genes of the Arabidopsis miR159 family. Proc Natl Acad Sci USA 104:16371–16376. doi:10.1073/pnas.0707653104
Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, Toriyama K (2003) A novel male-sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis-sensitive exine. Plant Mol Biol 53:107–116. doi:10.1023/B:PLAN.0000009269.97773.70
Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181. doi:10.1111/j.1365-313X.2004.02118.x
Ariizumi T, Kawanabe T, Hatakeyama K, Sato S, Kato T, Tabata S, Toriyama K (2008) Ultrastructural characterization of exine development of the transient defective exine 1 mutant suggests the existence of a factor involved in constructing reticulate exine architecture from sporopollenin aggregates. Plant Cell Physiol 49:58–67. doi:10.1093/pcp/pcm167
Beckmann R, Bubeck D, Grassucci R, Penczek P, Verschoor A, Blobel G, Frank J (1997) Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science 278:2123–2126. doi:10.1126/science.278.5346.2123
Boavida LC, McCormick S (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52:570–582. doi:10.1111/j.1365-313X.2007.03248.x
Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H (2006) Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol 140:1151–1168. doi:10.1104/pp.105.074708
Borg M, Brownfield L, Twell D (2009) Male gametophyte development: a molecular perspective. J Exp Bot. doi:10.1093/jxb/ern355
Brownfield L, Hafidh S, Borg M, Sidorova A, Mori T, Twell D (2009) A plant germ cell-specific integrator of cell cycle progression and sperm specification. PLoS Genet 5 doi:10.1371/journal.pgen.1000430
Chen YN, Slabaugh E, Brandizzi F (2008) Membrane-tethered transcription factors in Arabidopsis thaliana: novel regulators in stress response and development. Curr Opin Plant Biol 11:695–701. doi:10.1016/j.pbi.2008.10.005
Choi H, Hong J, Ha J, Kang J, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275:1723–1730. doi:10.1074/jbc.275.3.1723
Clough SJ, Bent AF (1998) Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. doi:10.1046/j.1365-313x.1998.00343.x
Cluis CP, Mouchel CF, Hardtke CS (2004) The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J 38:332–347. doi:10.1111/j.1365-313X.2004.02052.x
Correa LG, Riano-Pachon DM, Schrago CG, dos Santos RV, Mueller-Roeber B, Vincentz M (2008) The role of bZIP transcription factors in green plant evolution: adaptive features emerging from four founder genes. PLoS ONE 3:e2944. doi:10.1371/journal.pone.0002944
Darlington GJ, Wang N, Hanson RW (1995) C/EBP alpha: a critical regulator of genes governing integrative metabolic processes. Curr Opin Genet Dev 5:565–570. doi:10.1016/0959-437X(95)80024-7
Darlington GJ, Ross SE, MacDougald OA (1998) The role of C/EBP genes in adipocyte differentiation. J Biol Chem 273:30057–30060. doi:10.1074/jbc.273.46.30057
Deppmann CD, Alvania RS, Taparowsky EJ (2006) Cross-species annotation of basic leucine zipper factor interactions: insight into the evolution of closed interaction networks. Mol Biol Evol 23:1480–1492. doi:10.1093/molbev/msl022
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328. doi:10.1111/j.1365-313X.2005.02379.x
Dupl’áková N, Reňák D, Hovanec P, Honysová B, Twell D, Honys D (2007) Arabidopsis gene family profiler (aGFP)—user-oriented transcriptomic database with easy-to-use graphic interface. BMC Plant Biol 7:39. doi:10.1186/1471-2229-7-39
Durbarry A, Vizir I, Twell D (2005) Male germ line development in Arabidopsis. duo pollen mutants reveal gametophytic regulators of generative cell cycle progression. Plant Physiol 137:297–307. doi:10.1104/pp.104.053165
Eady C, Lindsey K, Twell D (1995) The significance of microspore division and division symmetry for vegetative cell-specific transcription and generative cell differentiation. Plant Cell 7:65–74
Eferl R, Sibilia M, Hilberg F, Fuchsbichler A, Kufferath I, Guertl B, Zenz R, Wagner EF, Zatloukal K (1999) Functions of c-Jun in liver and heart development. J Cell Biol 145:1049–1061. doi:10.1083/jcb.145.5.1049
Ehlert A, Weltmeier F, Wang X, Mayer CS, Smeekens S, Vicente-Carbajosa J, Droge-Laser W (2006) Two-hybrid protein–protein interaction analysis in Arabidopsis protoplasts: establishment of a heterodimerization map of group C and group S bZIP transcription factors. Plant J 46:890–900. doi:10.1111/j.1365-313X.2006.02731.x
Finkelstein RR, Lynch TJ (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12:599–609
Fukazawa J, Sakai T, Ishida S, Yamaguchi I, Kamiya Y, Takahashi Y (2000) Repression of shoot growth, a bZIP transcriptional activator, regulates cell elongation by controlling the level of gibberellins. Plant Cell 12:901–915
Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN (2008) RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol 147:852–863. doi:10.1104/pp.108.118026
Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132:640–652. doi:10.1104/pp.103.020925
Honys D, Twell D (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5:R85. doi:10.1186/gb-2004-5-11-r85
Honys D, Reňák D, Twell D (2006) Male gametophyte development and function. In: Teixeira da Silva J (ed) Floriculture, ornamental and plant biotechnology: advances and topical issues, 1st edn. Global Science Books, London, pp 76–87
Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K (2007) Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 19:3549–3562. doi:10.1105/tpc.107.054536
Iwata Y, Koizumi N (2005) An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc Natl Acad Sci USA 102:5280–5285. doi:10.1073/pnas.0408941102
Iwata Y, Fedoroff NV, Koizumi N (2008) Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 20(11):3107–3121
Jakoby M, Weisshaar B, Droge-Laser W, Vicente-Carbajosa J, Tiedemann J, Kroj T, Parcy F (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7:106–111. doi:10.1016/S1360-1385(01)02223-3
Karimi M, De Meyer B, Hilson P (2005) Modular cloning in plant cells. Trends Plant Sci 10:103–105
Kindl H (1993) Fatty acid degradation in plant peroxisomes: function and biosynthesis of the enzymes involved. Biochimie 75:225–230. doi:10.1016/0300-9084(93)90080-C
Leroch M, Neuhaus HE, Kirchberger S, Zimmermann S, Melzer M, Gerhold J, Tjaden J (2008) Identification of a novel adenine nucleotide transporter in the endoplasmic reticulum of Arabidopsis. Plant Cell 20:438–451. doi:10.1105/tpc.107.057554
Li C, Wong WH (2001a) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98:31–36. doi:10.1073/pnas.011404098
Li C, Wong WH (2001b) Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol 2:R32
Liu JX, Srivastava R, Che P, Howell SH (2007a) An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Plant Cell 19:4111–4119. doi:10.1105/tpc.106.050021
Liu JX, Srivastava R, Che P, Howell SH (2007b) Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J 51:897–909. doi:10.1111/j.1365-313X.2007.03195.x
Lu G, Gao C, Zheng X, Han B (2008) Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Epub ahead of print, Planta
Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Muller-Rober B, Schulz B (2002) Multifunctionality of plant ABC transporters—more than just detoxifiers. Planta 214:345–355. doi:10.1007/s004250100661
McCormick S (2004) Control of male gametophyte development. Plant Cell 16(Suppl):S142–S153. doi:10.1105/tpc.016659
Millar AA, Gubler F (2005) The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 17:705–721. doi:10.1105/tpc.104.027920
Murphy DJ (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40:325–438. doi:10.1016/S0163-7827(01)00013-3
Newman JR, Keating AE (2003) Comprehensive identification of human bZIP interactions with coiled-coil arrays. Science 300:2097–2101. doi:10.1126/science.1084648
Nijhawan A, Jain M, Tyagi AK, Khurana JP (2008) Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol 146:333–350. doi:10.1104/pp.107.112821
Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Preuss D (2005) Callose (beta-1, 3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5:22. doi:10.1186/1471-2229-5-22
Ohlrogge JB, Browse J, Somerville CR (1991) The genetics of plant lipids. Biochim Biophys Acta 1082:1–26
Park SK, Howden R, Twell D (1998) The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125:3789–3799
Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127:1739–1749. doi:10.1104/pp.010517
Piffanelli P, Ross JHE, Murphy DJ (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11:65–80. doi:10.1007/s004970050122
Pina C, Pinto F, Feijo JA, Becker JD (2005) Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol 138:744–756. doi:10.1104/pp.104.057935
Pracharoenwattana I, Cornah JE, Smith SM (2007) Arabidopsis peroxisomal malate dehydrogenase functions in beta-oxidation but not in the glyoxylate cycle. Plant J 50:381–390. doi:10.1111/j.1365-313X.2007.03055.x
Ringli C, Keller B (1998) Specific interaction of the tomato bZIP transcription factor VSF-1 with a non-palindromic DNA sequence that controls vascular gene expression. Plant Mol Biol 37:977–988. doi:10.1023/A:1006030007333
Rotman N, Durbarry A, Wardle A, Yang WC, Chaboud A, Faure JE, Berger F, Twell D (2005) A novel class of MYB factors controls sperm-cell formation in plants. Curr Biol 15:244–248. doi:10.1016/j.cub.2005.01.013
Sanchez-Fernandez R, Davies TG, Coleman JO, Rea PA (2001a) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 276:30231–30244. doi:10.1074/jbc.M103104200
Sanchez-Fernandez R, Rea PA, Davies TG, Coleman JO (2001b) Do plants have more genes than humans? Yes, when it comes to ABC proteins. Trends Plant Sci 6:347–348. doi:10.1016/S1360-1385(01)02038-6
Sanyal S, Sandstrom DJ, Hoeffer CA, Ramaswami M (2002) AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature 416:870–874. doi:10.1038/416870a
Schindler U, Menkens AE, Beckmann H, Ecker JR, Cashmore AR (1992) Heterodimerization between light-regulated and ubiquitously expressed Arabidopsis GBF bZIP proteins. EMBO J 11:1261–1273
Seo PJ, Kim SG, Park CM (2008) Membrane-bound transcription factors in plants. Trends Plant Sci 13(10):550–556
Shen H, Cao K, Wang X (2007) A conserved proline residue in the leucine zipper region of AtbZIP34 and AtbZIP61 in Arabidopsis thaliana interferes with the formation of homodimer. Biochem Biophys Res Commun 362:425–430. doi:10.1016/j.bbrc.2007.08.026
Shen H, Cao K, Wang X (2008) AtbZIP16 and AtbZIP68, two new members of GBFs, can interact with other G group bZIPs in Arabidopsis thaliana. BMB Rep 41:132–138
Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2:755–767
Svendsen A (2000) Lipase protein engineering. Biochim Biophys Acta 1543:223–238
Sze H, Padmanaban S, Cellier F, Honys D, Cheng NH, Bock KW, Conejero G, Li X, Twell D, Ward JM, Hirschi KD (2004) Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol 136:2532–2547. doi:10.1104/pp.104.046003
Tajima H, Iwata Y, Iwano M, Takayama S, Koizumi N (2008) Identification of an Arabidopsis transmembrane bZIP transcription factor involved in the endoplasmic reticulum stress response. Biochem Biophys Res Commun 374:242–247. doi:10.1016/j.bbrc.2008.07.021
Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N (1995) Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J 14:6193–6208
Takeda T, Amano K, Ohto MA, Nakamura K, Sato S, Kato T, Tabata S, Ueguchi C (2006) RNA interference of the Arabidopsis putative transcription factor TCP16 gene results in abortion of early pollen development. Plant Mol Biol 61:165–177. doi:10.1007/s11103-006-6265-9
Tateda C, Ozaki R, Onodera Y, Takahashi Y, Yamaguchi K, Berberich T, Koizumi N, Kusano T (2008) NtbZIP60, an endoplasmic reticulum-localized transcription factor, plays a role in the defense response against bacterial pathogens in Nicotiana tabacum. J Plant Res 121(6):603–611
Teller JK, Fahien LA, Valdivia E (1990) Interactions among mitochondrial aspartate aminotransferase, malate dehydrogenase, and the inner mitochondrial membrane from heart, hepatoma, and liver. J Biol Chem 265:19486–19494
Thimm O, Blasing O, Gibon Y, Nagel A, Meyer S, Kruger P, Selbig J, Muller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939. doi:10.1111/j.1365-313X.2004.02016.x
Twell D, Oh S-A, Honys D (2006) Pollen development, a genetic and transcriptomic view. In: Malho R (ed) The pollen tube, vol 3. Springer–Verlag, Berlin, Heidelberg, pp 15–45
Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97:11632–11637. doi:10.1073/pnas.190309197
Van Aelst AC, Pierson ES, Van Went JL, Cresti M (1993) Ultrastructural changes of Arabidopsis thaliana pollen during final maturation and rehydration. Zygote 1:173–179. doi:10.1017/S096719940000143X
Verelst W, Saedler H, Munster T (2007a) MIKC* MADS-protein complexes bind motifs enriched in the proximal region of late pollen-specific Arabidopsis promoters. Plant Physiol 143:447–460. doi:10.1104/pp.106.089805
Verelst W, Twell D, de Folter S, Immink R, Saedler H, Munster T (2007b) MADS-complexes regulate transcriptome dynamics during pollen maturation. Genome Biol 8:R249. doi:10.1186/gb-2007-8-11-r249
Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, Murphy A, Rea PA, Samuels L, Schulz B, Spalding EJ, Yazaki K, Theodoulou FL (2008) Plant ABC proteins—a unified nomenclature and updated inventory. Trends Plant Sci 13:151–159. doi:10.1016/j.tplants.2008.02.001
Vizcay-Barrena G, Wilson ZA (2006) Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J Exp Bot 57:2709–2717. doi:10.1093/jxb/erl032
Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF (1992) Bone and haematopoietic defects in mice lacking c-fos. Nature 360:741–745. doi:10.1038/360741a0
Watanabe Y, Yamamoto M (1996) Schizosaccharomyces pombe pcr1+ encodes a CREB/ATF protein involved in regulation of gene expression for sexual development. Mol Cell Biol 16:704–711
Weigel D, Glazebrook J (2002) Arabidopsis. A laboratory handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Weltmeier F, Rahmani F, Ehlert A, Dietrich K, Schutze K, Wang X, Chaban C, Hanson J, Teige M, Harter K, Vicente-Carbajosa J, Smeekens S, Droge-Laser W (2009) Expression patterns within the Arabidopsis C/S1 bZIP transcription factor network: availability of heterodimerization partners controls gene expression during stress response and development. Plant Mol Biol 69:107–119. doi:10.1007/s11103-008-9410-9
Wirtz KW (1991) Phospholipid transfer proteins. Annu Rev Biochem 60:73–99. doi:10.1146/annurev.bi.60.070191.000445
Xiang Y, Tang N, Du H, Ye H, Xiong L (2008) Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol 148:1938–1952. doi:10.1104/pp.108.128199
Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, Okamura H (2005) Role of DBP in the circadian oscillatory mechanism. Mol Cell Biol 20:4773–4781. doi:10.1128/MCB.20.13.4773-4781.2000
Yamamoto Y, Nishimura M, Hara-Nishimura I, Noguchi T (2003) Behavior of vacuoles during microspore and pollen development in Arabidopsis thaliana. Plant Cell Physiol 44:1192–1201. doi:10.1093/pcp/pcg147
Yang C, Vizcay-Barrena G, Conner K, Wilson ZA (2007) MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 19:3530–3548. doi:10.1105/tpc.107.054981
Yin Y, Zhu Q, Dai S, Lamb C, Beachy RNP (1997) RF2a, a bZIP transcriptional activator of the phloem-specific rice tungro bacilliform virus promoter, functions in vascular development. EMBO J 16:5247–5259. doi:10.1093/emboj/16.17.5247
Zhang ZB, Zhu J, Gao JF, Wang C, Li H, Zhang HQ, Zhang S, Wang DM, Wang QX, Huang H, Xia HJ, Yang ZN (2007) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J 52:528–538. doi:10.1111/j.1365-313X.2007.03254.x
Zhou SL, Stump D, Kiang CL, Isola LM, Berk PD (1995) Mitochondrial aspartate aminotransferase expressed on the surface of 3T3–L1 adipocytes mediates saturable fatty acid uptake. Proc Soc Exp Biol Med 208:263–270
Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN (2008) Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J 55:266–277. doi:10.1111/j.1365-313X.2008.03500.x
Zimmermann P, Hennig L, Gruissem W (2005) Gene-expression analysis and network discovery using Genevestigator. Trends Plant Sci 10:407–409. doi:10.1016/j.tplants.2005.07.003
Acknowledgments
Authors thank Dr Milada Čiamporová (Institute of Botany, Slovak Academy of Sciences) and Dr Aleš Soukup (Department of Plant Physiology, Charles University in Prague) for their expertise with evaluation of transmission electron micrographs. Authors gratefully acknowledge financial support from Grant Agency of the Czech Republic (GACR grants 522/06/0896 and 522/09/0858) and Ministry of Education of the Czech Republic (MSMT grant LC06004). DT acknowledges support from the Biotechnology and Biological Sciences Research Council (BBSRC) and the University of Leicester.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2009_9493_MOESM2_ESM.xls
Supplementary Table 2 Expression of AtbZIP transcription factors in various tissues and cell types according to aGFP database (Duplakova et al., 2007). (XLS 42 kb)
11103_2009_9493_MOESM5_ESM.xls
Supplementary table 5 List of genes showing late male gametophytic expression profile and at least two-fold downregulated in atbzip34 pollen (XLS 57 kb)
11103_2009_9493_MOESM6_ESM.xls
Supplementary Table 6 List of genes showing late male gametophytic expression profile and at least two-fold upregulated in atbzip34 pollen (XLS 23 kb)
11103_2009_9493_MOESM8_ESM.pdf
Supplementary Fig. 1 Transmission electron micrographs of cross-sections of developing male gametophyte from tetrad stage to bicellular pollen. wild type (A, C, E, G, I, K) and atbzip34 (B, D, F, H, J, L). Size bar corresponds to 2 µm (A, B, E-H, J-L) and 5 µm (C, D, I). Tetrad stage (A-D). Tetrads of haploid microspores are surrounded by callose wall and deposits of sporopollenin are visible on surface of outer callose wall in wt (A). Primexine forms characteristic undulations in wt, while the atbzip34 tetrads (B) seem to be younger with smooth plasma membrane and thinner callose wall with no sporopollenin deposits on the outher callosic wall. The structure of tapetum was similar in wt (C) and mutant (D). Uninucleate microspore stage (E-H). Microspores of wt (E) and mutant (F) looked similar with large nucleus and smooth cytoplasm. On the contrary, mutant tapetal cells (H) were less vacuolated than wt (G) and contained clusters of electron-dense granules along the locule wall. Late bicellular stage (I-L). Unlike wt (I), vegetative cell of atbzip34 bicellular pollen (J) was highly vacuolated and was enclosed in characteristic wrinkled intine On the contrary, there were no apparent ultrastructural differences in wt (K) and atbzip34 (L) tapetum; in both genotypes elaioplasts were fully developed. BA, baculae; C, callose wall; E, endothecium; EL, elaioplast; ER, endoplasmic reticulum; GC, generative cell; I, electron-dense inclusions; IN, intine; LO, anther locule; M, middle layer; MS, microspore; N, nucleus; P, plastid; S, starch; T, tapetum; TC, tectum; V, vacuole; VC vegetative cell (PDF 3107 kb)
11103_2009_9493_MOESM9_ESM.pdf
Supplementary Fig. 2 MapMan visualization of genes with altered expression in atbzip34 pollen. General transporters (A), genes involved in development and cell wall and lipid metabolism (B), stress-response genes (C) and metabolic pathways leading to cell wall precursors (C) are shown. Logarithmic scale; downregulated genes in blue, upregulated genes in red (PDF 581 kb)
11103_2009_9493_MOESM10_ESM.pdf
Supplementary Fig. 3 MapMan visualization of transcription factor genes with altered expression in atbzip34 pollen. Logarithmic scale; downregulated genes in blue, upregulated genes in red (PDF 245 kb)
Rights and permissions
About this article
Cite this article
Gibalová, A., Reňák, D., Matczuk, K. et al. AtbZIP34 is required for Arabidopsis pollen wall patterning and the control of several metabolic pathways in developing pollen. Plant Mol Biol 70, 581–601 (2009). https://doi.org/10.1007/s11103-009-9493-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-009-9493-y