Abstract
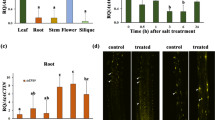
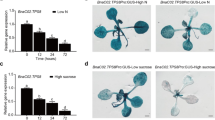
Rice plants (Oryza sativa L.) take up iron using iron-chelating compounds known as mugineic acid family phytosiderophores (MAs). In the biosynthetic pathway of MAs, nicotianamine aminotransferase (NAAT) catalyses the key step from nicotianamine to the 3′′-keto form. In the present study, we identified six rice NAAT genes (OsNAAT1–6) by screening a cDNA library made from Fe-deficient rice roots and by searching databases. Among the NAAT homologues, OsNAAT1 belongs to a subgroup containing barley functional NAAT (HvNAAT-A and HvNAAT-B) as well as a maize homologue cloned by cDNA library screening (ZmNAAT1). Northern blot and RT-PCR analysis showed that OsNAAT1, but not OsNAAT2–6, was strongly up-regulated by Fe deficiency, both in roots and shoots. The OsNAAT1 protein had NAAT enzyme activity in vitro, confirming that the OsNAAT1 gene encodes functional NAAT. Promoter–GUS analysis revealed that OsNAAT1 was expressed in companion and pericycle cells adjacent to the protoxylem of Fe-sufficient roots. In addition, expression was induced in all cells of Fe-deficient roots, with particularly strong GUS activity evident in the companion and pericycle cells. OsNAAT1 expression was also observed in the companion cells of Fe-sufficient shoots, and was clearly induced in all the cells of Fe-deficient leaves. These expression patterns highly resemble those of OsNAS1, OsNAS2 and OsDMAS1, the genes responsible for MAs biosynthesis for Fe acquisition. These findings strongly suggest that rice synthesises MAs in whole Fe-deficient roots to acquire Fe from the rhizosphere, and also in phloem cells to maintain metal homeostasis facilitated by MAs-mediated long-distance transport.




Similar content being viewed by others
Abbreviations
- DMA:
-
2′-Deoxymugineic acid
- GUS:
-
β-Glucuronidase
- MAs:
-
Mugineic acid family phytosiderophores
- NA:
-
Nicotianamine
References
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281:32395–32402
Colangelo EP, Guerinot ML (2006) Put the metal to petal: metal uptake and transport throughout plants. Curr Opin Plant Biol 9:322–330
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe 1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409:346–349
Herbik A, Koch G, Mock HP, Dushkov D, Czihal A, Thielmann J, Stephan UW, Baumlein H (1999) Isolation, characterization and cDNA cloning of nicotianamine synthase from barley. A key enzyme for iron homeostasis in plants. Eur J Biochem 265:231–239
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza Sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Higuchi K, Kanazawa K, Nishizawa NK, Mori S (1994) Purification and characterization of nicotianamine synthase from Fe deficient barley roots. Plant Soil 165:173–179
Higuchi K, Kanazawa K, Nishizawa NK, Mori S (1996) The role of nicotianamine synthase in response to Fe nutrition status in Gramineae. Plant Soil 178:171–177
Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S (1999) Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 119:471–479
Higuchi K, Watanabe S, Takahashi M, Kawasaki S, Nakanishi H, Nishizawa NK, Mori S (2001) Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions. Plant J 25:159–167
Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2003) Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J 36:366–381
Ishimaru Y, Kim S, Tsukamoto T, Oki H, Kobayashi T, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2007) Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc Natl Acad Sci USA 104:7373–7378
Kanazawa K, Higuchi K, Nishizawa NK, Fushiya S, Chino M, Mori S (1994) Nicotianamine aminotransferase activities are correlated to the phytosiderophore secretions under Fe-deficient conditions in Gramineae. J Exp Bot 45:1903–1906
Kanazawa K, Higuchi K, Nishizawa NK, Fushiya S, Mori S (1995) Detection of two distinct isozymes of nicotianamine aminotransferase in Fe-deficient barley roots. J Exp Bot 46:1241–1244
Kawai S, Itoh K, Takagi S, Nomoto K (1988) Studies on phytosiderophore: biosynthesis of mugineic acid and 2′-deoxymugineic acid in Hordeum vulgare L. var Minorimugi. Tetrahyd Lett 29:1053–1056
Kobayashi T, Nakanishi H, Takahashi M, Kawasaki S, Nishizawa NK, Mori S (2001) In vivo evidence that Ids3 from Hordeum vulgare encodes a dioxygenase that converts 2′-deoxymugineic acid to mugineic acid in transgenic rice. Planta 212:864–871
Kobayashi T, Nakayama Y, Itai RN, Nakanishi H, Yoshihara T, Mori S, Nishizawa NK (2003) Identification of novel cis-acting elements, IDE1 and IDE2, of the barley IDS2 gene promoter conferring iron-deficiency-inducible, root-specific expression in heterogeneous tobacco plants. Plant J 36:780–793
Kobayashi T, Nakayama Y, Takahashi M, Inoue H, Nakanishi H, Yoshihara T, Mori S, Nishizawa NK (2004) Construction of artificial promoters highly responsive to iron deficiency. Soil Sci Plant Nutr 50:1167–1175
Kobayashi T, Suzuki M, Inoue H, Itai RN, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2005) Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot 56:1305–1316
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J 39:415–424
Lytle CM, Jolley VD (1991) Iron deficiency stress response of various C-3 and C-4 grain crop genotypes: strategy II mechanism evaluated. J Plant Nutr 14:341–361
Ma JF, Nomoto K (1993) Two related biosynthetic pathways of mugineic acids in Gramineous plants. Plant Physiol 102:373–378
Ma JF, Shinada T, Matsuda C, Nomoto K (1995) Biosynthesis of phytosiderophores, mugineic acids, associated with methionine cycling. J Biol Chem 270:16549–16554
Ma JF, Taketa S, Chang YC, Iwashita T, Matsumoto H, Takeda K, Nomoto K (1999) Genes controlling hydroxylations of phytosiderophores are located on different chromosomes in barley (Hordeum vulgare L.). Planta 207:590–596
Mizuno D, Higuchi K, Sakamoto T, Nakanishi H, Mori S, Nishizawa NK (2003) Three nicotianamine synthase genes isolated from maize are differentially regulated by iron nutritional status. Plant Physiol 132:1989–1997
Mori S, Nishizawa N (1987) Methionine as a dominant precursor of phytosiderophores in Graminaceae plants. Plant Cell Physiol 28:1081–1092
Mori S, Nishizawa N, Hayashi H, Chino M, Yoshimura E, Ishihara J (1991) Why are young rice plants highly susceptible to iron deficiency? Plant Soil 130:143–156
Murata Y, Ma JF, Yamaji N, Ueno D, Nomoto K, Iwashita T (2006) A specific transporter for iron(III)-phytosiderophore in barley roots. Plant J 46:563–572
Nakanishi H, Okumura N, Umehara Y, Nishizawa NK, Chino M, Mori S (1993) Expression of a gene specific for iron deficiency (Ids3) in the roots of Hordeum vulgare. Plant Cell Physiol 34:401–410
Nakanishi H, Yamaguchi H, Sasakuma T, Nishizawa NK, Mori S (2000) Two dioxygenase genes, Ids3 and Ids2, from Hordeum vulgare are involved in the biosynthesis of mugineic acid family phytosiderophores. Plant Mol Biol 44:199–207
Negishi T, Nakanishi H, Yazaki J, Kishimoto N, Fujii F, Shimbo K, Yamamoto K, Sakata K, Sasaki T, Kikuchi S, Mori S, Nishizawa NK (2002) cDNA microarray analysis of gene expression during Fe-deficiency stress in barley suggests that polar transport of vesicles is implicated in phytosiderophore secretion in Fe-deficient barley roots. Plant J 30:83–94
Nozoye T, Inoue H, Takahashi M, Ishimaru Y, Nakanishi H, Mori S, Nishizawa NK (2007) The expression of iron homeostasis-related genes during rice germination. Plant Mol Biol 64:35–47
Ogo Y, Itai RN, Nakanishi H, Inoue H, Kobayashi T, Suzuki M, Takahashi M, Mori S, Nishizawa NK (2006) Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants. J Exp Bot 57:2867–2878
Ogo Y, Itai RN, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK (2007) The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J 51:366–377
Ohata T, Kanazawa K, Mihashi S, Nishizawa NK, Fushiya S, Nozoe S, Chino M, Mori S (1993) Biosynthetic pathway of phytosiderophores in iron-deficient Graminaceous plants. Development of an assay system for the detection of nicotianamine aminotransferase activity. Soil Sci Plant Nutr 39:745–749
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophore in roots of grasses. Plant Physiol 80:175–180
Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N (2004) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279:9091–9096
Shojima S, Nishizawa NK, Fushiya S, Nozoe S, Irifune T, Mori S (1990) Biosynthesis of phytosiderophores. In-vitro biosynthesis of 2′-deoxymugineic acid from l-methionine and nicotianamine. Plant Physiol 93:1497–1503
Suzuki M, Takahashi M, Tsukamoto T, Watanabe S, Matsuhashi S, Yazaki J, Kishimoto N, Kikuchi S, Nakanishi H, Mori S, Nishizawa NK (2006) Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J 48:85–97
Takagi S (1976) Naturally occuring iron-chelating compounds in oat- and rice-root washing. I. Activity measurement and preliminary characterization. Soil Sci Plant Nutr 22:423–433
Takagi S, Nomoto K, Takemoto S (1984) Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J Plant Nutr 7:469–477
Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa NK, Mori S (1999) Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol 121:947–956
Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S (2001) Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol 19:466–469
Takizawa R, Nishizawa NK, Nakanishi H, Mori S (1996) Effect of iron deficiency on S-adenosylmethionine synthetase in barley roots. J Plant Nutr 19:1189–1200
von Wirén N, Mori S, Marschner H, Römheld V (1994) Iron inefficiency in maize mutant ys1 (Zea mays L. cv yellow-stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol 106:71–77
von Wirén N, Klair S, Bansal S, Briat JF, Khodr H, Shioiri T, Leigh RA, Hider RC (1999) Nicotianamine chelates both Fe(III) and Fe(II). Implications for metal transport in plants. Plant Physiol 119:1107–1114
Acknowledgement
We thank Mr. Tatsuya Sakamoto for assistance with cloning of ZmNAAT.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, H., Takahashi, M., Kobayashi, T. et al. Identification and localisation of the rice nicotianamine aminotransferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Mol Biol 66, 193–203 (2008). https://doi.org/10.1007/s11103-007-9262-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-007-9262-8