Abstract
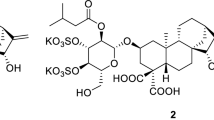
Alectrol was first isolated from root exudates of cowpea (Vigna unguiculata), a genuine host of a root parasitic weed Striga gesnerioides, as a germination stimulant for seeds of the parasite. The proposed structure, an isomer of strigol, was disproven by chemical synthesis. Recently, another structure, namely orobanchyl acetate, was proposed. Surprisingly, however, the synthetic compound having this proposed structure for alectrol was not active in inducing germination of S. gesnerioides seeds although it was active toward seeds of other root parasitic weeds such as S. hermonthica and Orobanche minor. Detailed studies on 1H NMR, mass and CD spectra of naturally occurring alectrol, re-isolated from cowpea root exudates, revealed that the genuine structure of the germination stimulant is not orobanchyl acetate but its stereoisomer ent-2′-epi-orobanchyl acetate. Accordingly, the structure of natural orobanchol was revised to ent-2′-epi-orobanchol 12 years after a tentative structure of orobanchol was proposed. Strict stereochemical requirements of strigolactones for germination induction of S. gesnerioides seeds, authentic samples of synthetic strigolactones and advanced analytical instruments made the structural assignment possible, thus ending a 20 years controversy concerning the true structure of alectrol.









Similar content being viewed by others
References
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Akiyama K, Ogasawara S, Ito S, Hayashi H (2010) Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol 51:1104–1117
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P et al (2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351
Brooks DW, Bevinakatti HS, Kennedy E, Hathaway J (1985) The absolute structure of (+)-strigol. J Org Chem 50:628–632
Brown R, Johnson AW, Robinson E, Tyler GJ (1952a) The Striga germination factor. II. Chromatographic purification of crude concentrates. Biochem J 50:596–600
Brown R, Johnson AW, Robinson E, Tyler GJ (1952b) The Orobanche germination factor. 3. Concentration of the factor by counter-current distribution. Biochem J 52:571–574
Butler LG (1995) In: Dakshini KMM, Einhelling EA (eds) Allelopathy: organisms, processes, and applications. American Chemical Society, Washington, pp 158–168
Chen VX, Boyer FD, Rareau C, Retailleau P, Vors JP, Beau MJ (2010) Stereochemistry, total synthesis, and biological evaluation of the new plant hormone solanacol. Chemistry 16:13941–13945
Cook CE, Whichard LP, Turner B, Wall ME, Egley GH (1966) Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154:1189–1190
Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P, Luhan PA, McPhail AT (1972) Germination stimulants. II. The structure of strigol—a potent seed germination stimulant for witchweed (Striga lutea Lour). J Am Chem Soc 94:6198–6199
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P et al (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Hauck C, Müller S, Shildknecht S (1992) A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J Plant Physiol 139:474–478
Heide- Jørgensen HS (2008) Parasitic flowering plants. Brill NV, Leiden
Hirayama K, Mori K (1999) Synthesis of (+)-strigol and (+)-orobanchol, the germination stimulants, and their stereoisomers by employing lipase-catalyzed asymmetric acetylation as the key step. Eur J Org Chem 1999(9):2211–2217
Matsui J, Bando M, Kido M, Takeuchi Y, Mori K (1999a) Synthetic disproof of the structure proposed for alectrol, the germination stimulant from Vigna unguiculata. Eur J Org Chem 1999(9):2195–2199
Matsui J, Yokota T, Bando M, Takeuchi Y, Mori K (1999b) Synthesis and structure of orobanchol, the germination stimulant for Orobanche minor. Eur J Org Chem 1999(9):2201–2210
Matsuura H, Ohashi K, Sasako H, Tagawa N, Takano Y, Ioka Y et al (2008) Germination stimulants from root exudates of Vigna unguiculata. Plant Growth Regul 54:31–36
Mori K, Matsui J, Bando M, Kido M, Takeuchi Y (1998) Synthetic disproof against the structure proposed for alectrol, the germination stimulant from Vigna unguiculata. Tetrahedron Lett 39:6023–6026
Motonami N, Ueno K, Nakashima H, Nomura S, Mizutani M, Takikawa H, Sugimoto Y (2013) Bioconversion of 5-deoxystrigol to sorgomol by the sorghum, Sorghum bicolor (L.) Moench. Phytochemistry 93:41–48
Müller S, Hauck C, Shildknecht S (1992) Germination stimulants produced by Vigna unguiculata Walp cv Saunders Upright. J Plant Growth Regul 11:77–84
Nomura S, Nakashima H, Mizutani M, Takikawa H, Sugimoto Y (2013) Structural requirements of strigolactones for germination induction and inhibition of Striga gesnerioides seeds. Plant Cell Rep 32:829–838
Parker C (2009) Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag Sci 65:453–459
Parker C (2012) Parasitic weeds: a world challenge. Weed Sci 60:269–276
Seto Y, Kameoka H, Yamaguchi S, Kyozuka J (2012) Recent advances in strigolactone research: chemical and biological aspects. Plant Cell Physiol 53:1843–1853
Seto Y, Sado A, Asami K, Hanada A, Umehara M, Akiyama K, Yamaguchi S (2014) Carlactone is an entogenous biosynthetic precursor for strigolactones. Proc Natl Acad Sci USA 111:1640–1645
Siame BP, Weerasuriya Y, Wood K, Ejeta G, Butler LG (1993) Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. J Agric Food Chem 41:1486–1491
Sugimoto Y, Wigchert SCM, Thuring JWJF, Zwanenburg B (1998) Synthesis of all eight stereoisomers of the germination stimulant sorgolactone. J Org Chem 63:1259–1267
Takikawa H, Jikumaru S, Sugimoto Y, Xie X, Yoneyama K, Sasaki M (2009) Synthetic disproof of the structure proposed for solanacol, the germination stimulant for seeds of root parasitic weeds. Tetrahedron Lett 50:4549–4551
Tsuchiya Y, McCourt P (2009) Strigolactones: a new hormone with a past. Curr Opin Plant Biol 12:556–561
Ueno K, Fujiwara M, Nomura S, Mizutani M, Sasaki M, Takikawa H, Sugimoto Y (2011a) Structural requirements of strigolactones for germination induction of Striga gesnerioides seeds. J Agric Food Chem 59:9226–9231
Ueno K, Nomura S, Muranaka S, Mizutani M, Takikawa H, Sugimoto Y (2011b) Ent-2′-epi-Orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover. J Agric Food Chem 59:10485–10490
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N et al (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Welzel P, Rohrig S, Milkova Z (1999) Strigol-type germination stimulants: the C-2′ configuration problem. Chem Commun 20:2017–2022
Wigchert SCM, Kuiper E, Boelhouwer GH, Nefkens GHL, Verkleij JAC, Zwanenburg B (1999) Dose-response of seeds of the parasitic weeds, Striga and Orobanche toward the synthetic germination stimulants GR 24 and Nijmegen 1. J Agric Food Chem 47:1705–1710
Xie X, Kusumoto D, Takeuchi Y, Yoneyama K, Yamada Y, Yoneyama K (2007) 2′-epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco. J Agric Food Chem 55:8067–8072
Xie X, Yoneyama K, Kusumoto D, Yamada Y, Yokota T, Takeuchi Y, Yoneyama K (2008a) Isolation and identification of alectrol as (+)-orobanchyl acetate, a germination stimulant for root parasitic plants. Phytochemistry 69:427–431
Xie X, Yoneyama K, Kusumoto D, Yamada Y, Takeuchi Y, Sugimoto Y, Yoneyama K (2008b) Sorgomol, germination stimulant for root parasitic plants, produced by Sorghum bicolor. Tetrahedron Lett 49:2066–2068
Xie X, Yoneyama K, Hanada Y, Fusegi N, Yamada Y, Ito S et al (2009) Fabacyl acetate, a germination stimulant for root parasitic plants from Pisum sativum. Phytochemistry 70:211–215
Xie X, Yoneyama K, Kisugi T, Uchida K, Ito S, Akiyama K et al (2013) Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol Plant 6:153–163
Yokota T, Sakai H, Okuno K, Yoneyama K, Takeuchi Y (1998) Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 49:1967–1973
Zwanenburg B, Pospíšil T (2013) Structure and activity of strigolactones: new plant hormones with a rich future. Mol Plant 6:38–62
Zwanenburg B, Mwakaboko AS, Reizelman A, Anilkumar G, Sethumadhavan D (2009) Structure and function of natural and synthetic signaling molecules in parasitic weed germination. Pest Manag Sci 65:478–491
Acknowledgments
We thank Emeritus professor Kenji Mori (The University of Tokyo, Japan) for the generous gifts of the synthetic orobanchol isomers, orobanchol (9), 2′-epi-orobanchol (2′-epi-9), 4-epi-orobanchol (4-epi-9) and 4,2′-bisepi-orobanchol (4,2′-bisepi-9). The work was supported, in part, by grants from the Asia Africa Science Platform Program of the Japan Society for the Promotion of Science, JST/JICA, Science and Technology Research Partnership for Sustainable Development (SATREPS), and Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (Nos. 11J01777, 23405023, 24658111 and 25292065).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueno, K., Sugimoto, Y. & Zwanenburg, B. The genuine structure of alectrol: end of a long controversy. Phytochem Rev 14, 835–847 (2015). https://doi.org/10.1007/s11101-014-9380-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-014-9380-2