Abstract
Key message
Structure–activity relationship studies of strigolactones and Striga gesnerioides seed germination revealed strict structural requirements for germination induction and a new function of the plant hormones as germination inhibitors.
Abstract
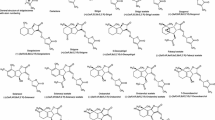
Stereoisomers of the naturally occurring strigolactones, strigol, sorgolactone, orobanchol, sorgomol and 5-deoxystrigol, 36 in total, were prepared and screened for the ability to induce and/or inhibit the germination of Striga hermonthica and Striga gesnerioides seeds collected from mature plants that parasitized on sorghum and cowpea, respectively. All of the compounds induced S. hermonthica seed germination, albeit displayed differential activities. On the other hand, only a limited number of the compounds induced significant germination in S. gesnerioides, thus indicating strict structural requirements. Strigolactones inducing high germination in S. gesnerioides induced low germination in S. hermonthica. Strigolactones with the same configuration at C3a, C8b and C2′ as that in 5-deoxystrigol (9a) induced high germination of S. hermonthica seeds, but most of them inhibited the germination of S. gesnerioides. The differential response of S. gesnerioides to strigolactones may play an important role in the survival of the species. However, the compounds could be used as means of control if mixed cropping of cowpea and sorghum is adopted.






Similar content being viewed by others
References
Akiyama K, Ogasawara S, Ito S, Hayashi H (2010) Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol 51:1104–1117
Babiker AGT, Ibrahim NE, Edwards WG (1988) Persistence of GR7 and Striga germination stimulant(s) from Euphorbia aegyptiaca Boiss in soils and in solutions. Weed Res 28:1–6
Bebawi FF, Eplee RE, Harris CE, Norris RS (1984) Longevity of witchweed (Striga asiatica) seed. Weed Sci 32:494–497
Brooks DW, Bevinakatti HS, Kennedy E, Hathaway J (1985) Practical Total Synthesis of (±)-Strigol. J Org Chem 50:628–632
Butler LG (1995) Chemical communication between the parasitic weed Striga and its crop host–A new dimension in allelochemistry. Allelopathy—Organisms, Processes, and Application Chapter 12, ACS Symposium Series, vol 582, pp 158–168
Cardwell KF, Jane JA (1995) Effect of soils, cropping system and host phenotype on incidence and severity of Striga gesnerioides on cowpea in West Africa. Agricult Ecosyst Environ 53:253–262
Cook CE, Whichard LP, Turner B, Wall ME, Egley GH (1966) Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154:1189–1190
Evidente A, Fernández-Aparicio M, Cimmino A, Rubiales D, Andolfi A, Motta A (2009) Peagol and peagoldione, two new strigolactone-like metabolites isolated from pea root exudates. Tetrahedron Lett 50:6955–6958
Fernández-Aparicio M, Flores F, Rubiales D (2009) Recognition of root exudates by seeds of broomrape (Orobanche and Phelipanche) species. Ann Bot 103:423–431
Fernández-Aparicio M, Yoneyama K, Rubiales D (2011) The role of strigolactones in host specificity of Orobanche and Phelipanche seed germination. Seed Sci Res 21:55–61
Frischmuth K, Samson E, Kranz A, Welzel P, Meuer H, Sheldrick WS (1991) Routes to derivatives of strigol (the witchweed germination factor) modified in the 5-position. Tetrahedron 47:9793–9806
Gibot-Leclerc S, Corbineau F, Sallé G, Côme D (2004) Responsiveness of Orobanche ramosa L. seeds to GR 24 as related to temperature, oxygen availability and water potential during preconditioning and subsequent germination. Plant Growth Regul 43:63–71
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Hauck C, Müller S, Schildknecht H (1992) A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J Plant Physiol 139:474–478
Hearne SJ (2009) Control: the Striga conundrum. Pest Manag Sci 65:603–614
Hirayama K, Mori K (1999) Synthesis of (+)-strigol and (+)-orobanchol, the germination stimulants, and their stereoisomers by employing lipase-catalyzed asymmetric acetylation as the key step. Eur J Org Chem 2211–2217
Hooper AM, Hassanali A, Chamberlain K, Khan Z, Pickett JA (2009) New genetic opportunities from legume intercrops for controlling Striga spp. parasitic weeds. Pest Manag Sci 65:546–552
Igbinnosa I, Okonkwo SNC (1992) Stimulation of germination of seeds of cowpea witchweed (Striga gesnerioides) by sodium hypochlorite and some growth regulators. Weed Sci 40:25–28
Johnson AW, Rosebery G, Parker C (1976) A novel approach to Striga and Orobanche control using synthetic germination stimulants. Weed Res 16:223–227
Johnson AW, Gowada G, Hassanali A, Knox J, Monaco S, Razavi Z, Rosebery G (1981) The preparation of synthetic analogues of strigol. J Chem Soc Perkin Trans 1:1734–1743
Kahn ZR, Hassanali H, Overholt W, Khamis TM, Hooper AM, Pickett JA, Wadhams LJ, Woodcock CM (2002) Control of witchweed Striga hermonthica by inter cropping with Desmodium spp., and the mechanism defined as allelopathic. J Chem Ecol 28:1871–1885
Kgosi RL, Zwanenburg B, Mwakaboko AS, Murdoch AS (2012) Strigolactone analogues induce suicidal seed germination of Striga spp. in soil. Weed Res 52:197–203
Kitahara S, Tashiro T, Sugimoto Y, Sasaki M, Takikawa H (2011) First synthesis of (±)-sorgomol, the germination stimulant for root parasitic weeds isolated from Sorghum bicolor. Tetrahedron Lett 52:724–726
Kondo Y, Tadokoro E, Matsuura M, Iwasaki K, Sugimoto Y, Miyake H, Takikawa H, Sasaki M (2007) Synthesis and seed germination stimulating activity of some imino analogs of strigolactones. Biosci Biotechnol Biochem 71:2781–2786
Malik H, Kohlen W, Jamil M, Rutjes FPJT, Zwanenburg B (2011) Aromatic A-ring analogues of orobanchol, new germination stimulants for seeds of parasitic weeds. Org Biomol Chem 9:2286–2293
Mangnus EM, Dommerholt FJ, De Jong RLP, Zwanenburg B (1992) Improved synthesis of strigol analogue GR24 and evaluation of the biological activity of its diastereomers. J Agric Food Chem 40:1230–1235
Matsui J, Yokota T, Bando M, Takeuchi Y, Mori K (1999) Synthesis and structure of orobanchol, the germination stimulant for Orobanche minor. Eur J Org Chem 2201–2210
Müller S, Hauck C, Schildknecht H (1992) Germination stimulants produced by Vigna unguiculata Walp cv Saunders Upright. J Plant Growth Regul 11:77–84
Muranaka S, Fatokun C, Boukar O (2011) Stability of Striga gesnerioides resistance mechanism in cowpea under high infestation level, low soil fertility and drought stresses. J Food Agric Environ 9:313–318
Mwakaboko AS, Zwanenburg B (2011) Strigolactone analogs derived from ketones using a working model for germination stimulants as a blueprint. Plant Cell Physiol 52:699–715
Oseni TO (2010) Evaluation of sorghum-cowpea intercrop productivity in Savanna agro-ecology using completion indices. J Agric Sci 2:229–234
Parker C (2009) Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag Sci 65:453–459
Reizelman A, Scheren M, Nefkens GHL, Zwanenburg B (2000) Synthesis of all eight stereoisomers of the germination stimulant strigol. Synthesis 1944–1951
Rubiales D, Fernández-Aparicio M, Wegmann K, Joel DM (2009) Revisiting strategies for reducing the seedbank of Orobanche and Phelipanche spp. Weed Res 49:23–33
Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Kleeb HJ (2005) The decreased apical dominance1/Petunia hybrid CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, Root Growth, and Flower Development. Plant Cell 17:746–759
Sugimoto Y, Wighcert SCM, Thuring JWJF, Zwanenburg B (1998) Synthesis of all eight stereoisomers of the germination stimulant sorgolactone. J Org Chem 63:1259–1267
Sugimoto Y, Ali AM, Yabuta S, Kinoshita H, Inanaga S, Itai A (2003) Germination strategy of Striga hermonthica involves regulation of ethylene biosynthesis. Physiol Plant 119:137–145
Thuring JWJF, Nefkens GHL, Zwanenburg B (1997) Asymmetric synthesis of all stereoisomers of the strigol analogue GR24. Dependence of absolute configuration on stimulatory activity of Striga hermonthica and Orobanche crenata Seed germination. J Agric Food Chem 45:2278–2283
Ueno K, Fujiwara M, Nomura S, Mizutani M, Sasaki M, Takikawa H, Sugimoto Y (2011a) Structural requirements of strigolactones for germination induction of Striga gesnerioides Seeds. J Agric Food Chem 59:9226–9231
Ueno K, Nomura S, Muranaka S, Mizutani M, Takikawa H, Sugimoto Y (2011b) Ent-2′-epi-orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover. J Agric Food Chem 59:10485–10490
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Xie X, Yoneyama K, Kusumoto D, Yamada Y, Yokota T, Takeuchi Y, Yoneyama K (2008) Isolation and identification of alectrol as (+)-orobanchyl acetate, a germination stimulant for root parasitic plants. Phytochemistry 69:427–431
Yokota T, Sakai H, Okuno K, Yoneyama K, Takeuchi Y (1998) Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 49:1967–1973
Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y (2010) Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol 51:1095–1103
Zwanenburg B, Pospíšil T (2013) Structure and activity of strigolactones, new plant hormons with a rich future. Mol Plant 6:38–62
Zwanenburg B, Mwakaboko AS, Reizelman A, Anikumar G, Sethumadhavan D (2009) Structure and function of natural and synthetic signaling molecules in parasitic weed germination. Pest Manag Sci 65:478–491
Acknowledgements
The authors are grateful to Prof. Abdel Gabar Babiker for critical reading of the manuscript. This study was supported by grants from JST/JICA, Science and Technology Research Partnership for Sustainable Development (SATREPS) and Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (Nos. 23405023 and 24658111).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar.
A contribution to the Special Issue: Plant Hormone Signaling.
Rights and permissions
About this article
Cite this article
Nomura, S., Nakashima, H., Mizutani, M. et al. Structural requirements of strigolactones for germination induction and inhibition of Striga gesnerioides seeds. Plant Cell Rep 32, 829–838 (2013). https://doi.org/10.1007/s00299-013-1429-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1429-y