Abstract
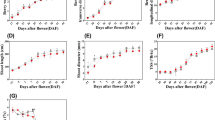
The effect of different growth regulators on growth and the production of terpenoid indole alkaloids as well as some enzymes involved in the biosynthesis were studied in Catharanthus roseus seedlings. The seedlings were grown on MS solid medium containing different concentrations of each growth regulator for a period of one month. Extracted alkaloids were analyzed by HPLC for determination of terpenoid indole alkaloid quantities. Continuous availability of growth regulators induced different alkaloids with variable effects among the regulators. Gibberellic acid at concentration of either 5.8 μM or 11.6 μM resulted in elongation of shoots with lowering the number of leaves. Abscisic acid has a retardant effect on growth. Ethylene did not effect the growth pattern at concentration of 100 μM but seedlings were not tolerant to higher concentrations. Methyljasmonate reduced the growth of the root system. Methyljasmonate was a general inducer for all alkaloids and increased the activity of strictosidine glucosidase. Ethylene applications promoted the pathways towards ajmalicine, serpentine, tabersonine and vindoline. Similar effect as for ethylene was observed for abscisic acid. Salicylic acid treatment increased the production of serpentine, tabersonine and higher concentration of salicylic acid induced vindoline accumulation. Peroxidase activity was also induced by salicylic acid. Gibberellic acid has little effect on alkaloid levels.
Similar content being viewed by others
References
Aerts R.J., Gisi D., De Carolis E, De Luca V. and Baumann T.W. 1994. Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus roseus and Cinchona seedlings. Plant J. 5: 635–643.
Aerts R.J., Schafer A., Hesse M., Baumann T.W. and Slusarenko A. 1996. Signalling molecules and the synthesis of alkaloids in Catharanthus roseus seedlings. Phytochemistry 42: 417–422.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
De Luca V., Balsevich J., Tyler R.T., Eilert U., Panchuk B.D. and Kurz W.G.W. 1986. Biosynthesis of indole alkaloids: developmental regulation of the biosynthetic pathway from tabersonine to vindoline in Catharanthus roseus. J. Plant Physiol. 125: 147–156.
De Luca V., Fernandez J.A., Campbell D. and Kurz W.G.W. 1988. Developmental regulation of enzymes of indole alkaloid biosynthesis in Catharanthus roseus. J. Plant Physiol. 86: 447–450.
El-Sayed M. and Verpoorte R. 2002. Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tiss. Org. Cult. 68: 265–270.
Geerlings A., Memelink J., van der Heijden R. and Verpoorte R. 2000. Molecular cloning and analysis of strictosidine β-D-glucosidase, an enzyme in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J. Biol. Chem. 275: 3051–3056.
Godoy-Hernandez G. and Loyola-Vargas V.M. 1997. Effect of acetylsalicylic acid on secondary metabolism of Catharanthus roseus tumor suspension cultures. Plant Cell Rep. 16: 287–290.
Kang S., Jung H., Kang Y., Yun D., Bahk J., Yang J. and Choi M. 2004. Effects of methyl jasmonate and salicylic acid on the production of tropane alkaloids and the expression of PMT and H6H in adventitious root cultures of Scopolia parviflora. Plant Sci. 166: 745–751.
Kargi F 1988. Alkaloids formation by Catharanthus roseus cells in a packed column biofilm bioreactor. Biotechnol. Lett. 10: 181–186.
Lee K., Hirano H., Yamakawa T., Kodama T., Igarashi Y. and Shimomura T. 2001. Responses of transformed root cultures of Atropa belladonna to salicylic acid stress. J. Biosci. Bioeng. 91: 586–589.
Luijendijk T., Stevens L.H. and Verpoorte R. 1998. Purification and characterization of strictosidine β-D-glucosidase from Catharanthus roseus cell suspension cultures. Plant Physiol. Biochem. 36: 419–425.
Mahady G.B., Liu C. and Beecher C.W. 1998. Involvement of protein kinase and G proteins in the signal transduction of benzophenanthridine alkaloid biosynthesis. Phytochemistry 48: 93–102.
Maehly A.C. and Chance B. 1954. The assay of catalases and peroxidases. Meth. Biochem. Anal. 1: 357–424.
Menke F.L.H., Parchmann S., Mueller M.J., Kijne J.W. and Memelink J. 1999. Involvement of the Octadecanoid Pathway and Protein Phosphorylation in Fungal Elicitor-Induced Expression of Terpenoid Indole Alkaloid Biosynthetic Genes in Catharanthus roseus. Plant Physiol. 119: 1289–1296.
Murashige T. and Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 15: 473–497.
Smith J.I., Smart N.J., Kurz W.G.W. and Misawa M. 1987. Stimulation of indole alkaloid production in cell suspension cultures of Catharanthus roseus by abscisic acid. Planta Med. 53: 470–474.
Stevens L.H., Schripsema J., Pennings E.J.M. and Verpoorte R. 1992. Activities of enzymes involved in indole alkaloid bio-synthesis in suspension cultures of Catharanthus, Cinchona and Tabernaemontana species. Plant Physiol. Biochem. 30: 675–681.
Vázqyuez-Flota F. and De Luca V. 1998. Jasmonate modulates development-and light-regulated alkaloid biosynthesis in Catharanthus roseus.. Phytochemistry 49: 395–402.
Verpoorte R., van der Heijden R., Van Gulik W.M. and ten Hoopen H.J.G. 1991. Plant biotechnology for the production of alkaloids: present and prospects. In: Brossi A. <nt>(ed.)</nt>, The Alkaloids. Vol. 40. Academic Press, San Diego.
Verpoorte R., van der Heijden R. and Moreno P.R.H. 1997. Biosynthesis of terpenoid indole alkaloids in Catharanthus roseus cells. In: Cordell G.A. <nt>(ed.)</nt>, The Alkaloids Vol. 49. Academic Press, pp. 221–299.
Yahia A., Kevers C., Gaspar T., Chénieux J., Rideau M. and Créche J. 1998. Cytokinins and ethylene stimulate indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus by two distinct mechanisms. Plant Sci. 133: 9–15.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Sayed, M., Verpoorte, R. Growth, metabolic profiling and enzymes activities of Catharanthus roseus seedlings treated with plant growth regulators. Plant Growth Regulation 44, 53–58 (2004). https://doi.org/10.1007/s10725-004-2604-5
Issue Date:
DOI: https://doi.org/10.1007/s10725-004-2604-5