Abstract
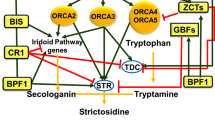
Precursor feeding experiments have shown that the terpene moiety biosynthesis is the most rate-limiting step of terpenoid indole alkaloids (TIA) produced by Catharanthus roseus suspension cells. The biosynthesis of TIA terpene moiety is strictly regulated by hormones: auxin inhibits, jasmonate stimulates and cytokinin and ethylene enhance their biosynthesis. Biochemical analyses coupled to molecular approaches have outlined a regulatory cross-talk between two metabolite pathways leading to the biosynthesis of terpene precursors. Terpenes derivatives produced through the mevalonic acid (MVA) pathway regulate the activity of the 2-C-methyl-d-erythritol 4-phosphate (MEP) non-mevalonate pathway producing the precursor of the TIA terpene moiety. This cross-talk involves regulatory prenylated proteins and acts on the regulation of the expression of early steps of monoterpenoid biosynthesis (ESMB) genes. The expression of the genes involved in the TIA terpene moiety biosynthesis is regulated in part by a general TIA transcription factor, ORCA3, and other unidentified transcription factors. This review sums up the essential information obtained at the metabolic, physiological, biochemical and molecular levels of regulation of TIA terpene moiety biosynthesis in C. roseus. We propose a synthetic scheme of the general regulatory network emerging from these experimental data, and discuss the related hypothesis and prospect in elucidating other key events involved in the regulation of TIA terpene moiety biosynthesis.

Similar content being viewed by others
Abbreviations
- CaaX-PTases:
-
CaaX-prenyltransferases
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- DMAPP:
-
Dimethylallyl diphosphate
- ESMB genes:
-
Early steps of monoterpenoid biosynthesis genes
- IPP:
-
Isopentenyl diphosphate
- MeJa:
-
Methyl Jasmonate
- MEP:
-
2-C-methyl-d-erythritol 4-phosphate
- MVA:
-
Mevalonic acid
- ORCA3:
-
Octadecanoid-derivative Responsive Catharanthus AP2-domain protein 3
- PFT:
-
Protein farnesyltransferase
- PGGT-I:
-
Type I protein geranylgeranyltransferase
- RabGGTase:
-
Rab geranylgeranyltransferase
- TIA:
-
Terpenoid indole alkaloids
References
Aapro MS, Harper P, Johnson SA, Vermorken JB (2001) Developments in cytotoxic chemotherapy: advances in treatment utilising vinorelbine. Crit Rev Oncol Hematol 40:251–63
Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH (1997) Terpenoid biosynthesis from 1-deoxy-D-xylulose in higher plants by intramolecular skeletal rearrangement. Proc Natl Acad Sci USA 94:10600–10605
Arvy MP, Imbault N, Naudascher F, Thiersault M, Doireau P (1994) 2,4-D and alkaloid accumulation in periwinkle cell suspensions. Biochimie 76:410–416
Bloch K, Chaykin S, Phillips AH, De Waard A (1959) Mevalonic acid pyrophosphate and isopentenylpyrophosphate. J Biol Chem 234:2595–2604
Burlat V, Oudin A, Courtois M, Rideau M, St-Pierre B (2004) Co-expression of three MEP pathway genes and geraniol 10-hydroxylase in internal phloem parenchyma of Catharanthus roseus implicates multicellular translocation of intermediates during the biosynthesis of monoterpene indole alkaloids and isoprenoid-derived primary metabolites. Plant J 38:131–141
Canel C, Lopes-Cardoso MI, Whitmer S, van der Fits L, Pasquali G, van der Heijden R, Hoge JH, Verpoorte R (1998) Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419
Carew DP, Krueger RJ (1977) Catharanthus roseus tissue culture: the effects of medium modifications on growth and alkaloid production. Lloydia 40:326–336
Chahed K, Oudin A, Guivarc’h N, Hamdi S, Chénieux JC, Rideau M, Clastre M (2000) 1-Deoxy-D-xylulose 5-phosphate synthase from perwinkle: cDNA identification and induced gene expression in terpenoid indole alkaloid-producing cells. Plant Physiol Biochem 38:559–566
Collu G, Unver N, Peltenburg-Looman AM, van der Heijden R, Verpoorte R, Memelink J (2001) Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett 508:215–220
Contin A, Heijden R, Lefeber AWM, Verpoort R (1998) The iridoid glucoside secologanin is derived from the novel triose phosphate/pyruvate pathway in a Catharanthus roseus cell culture. FEBS Lett 437:413–416
Courdavault V, Burlat V, St-Pierre B, Giglioli-Guivarc’h N (2005) Characterisation of CaaX-prenyltransferases in Catharanthus roseus: relationships with the expression of genes involved in the early stages of monoterpenoid biosynthetic pathway. Plant Sci 168:1097–1107
Courdavault V, Thiersault M, Courtois M, Gantet P, Oudin A, Doireau P, St-Pierre B, Giglioli-Guivarc’h N (2005) CaaX-prenyltransferases are essential for expression of genes involved in the early stages of monoterpenoid biosynthetic pathway in Catharanthus roseus cells. Plant Mol Biol 57:855–870
Décendit A, Liu D, Ouelhazi L, Doireau P, Mérillon J, Rideau M (1992) Cytokinin-enhanced accumulation of indole alkaloids in Catharanthus roseus cell cultures – the factor affecting the cytokinin response. Plant Cell Rep 11:400–403
Doller G, Alfermann AW, Reinhard E (1976) Production of indole alkaloids in tissue cultures of Catharanthus roseus. Planta Med 30:14–20
Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 6:78–84
Facchini PJ, DiCosmo F (1991) Secondary metabolite biosynthesis in cultured cells of Catharanthus roseus (L.) G. Don immobilized by adhesion to glass fibres. Appl Microbiol Biotechnol 35:382–392
Gadbut AP, Wu L, Tang D, Papageorge A, Watson JA, Galper JB (1997) Induction of the cholesterol metabolic pathway regulates the farnesylation of RAS in embryonic chick heart cells: a new role for ras in regulating the expression of muscarinic receptors and G proteins. EMBO J 16:7250–7260
Gantet P, Imbault N, Thiersault M, Doireau P (1998) Necessity of a functionnal octadecanoic pathway for indole alkaloid synthesis by Catharanthus roseus cell suspension cultured in an auxin-starved medium. Plant Cell Physiol 39:220–225
Gantet P, Memelink J (2002) Transcription factors: tools to engineer the production of pharmacologically active plant metabolites. Trends Pharmacol Sci 23:563–569
Goddijn OJ, Pennings EJ, van der Helm P, Schilperoort RA, Verpoorte R, Hoge JH (1995) Overexpression of a tryptophan decarboxylase cDNA in Catharanthus roseus crown gall calluses results in increased tryptamine levels but not in increased terpenoid indole alkaloid production. Transgenic Res 4:315–323
Hemmerlin A, Hoeffler JF, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J Biol Chem 278:26666–26676
Hughes EH, Hong SB, Gibson SI, Shanks JV, San KY (2004) Expression of a feedback-resistant anthranilate synthase in Catharanthus roseus hairy roots provides evidence for tight regulation of terpenoid indole alkaloid levels. Biotechnol Bioeng 86:718–727
Hughes EH, Hong SB, Gibson SI, Shanks JV, San KY (2004) Metabolic engineering of the indole pathway in Catharanthus roseus hairy roots and increased accumulation of tryptamine and serpentine. Metab Eng 6:268–276
Imbault N, Thiersault M, Dupéron P, Benabdelmouna A, Doireau P (1996) Pravastatine: A tool for investigating the availability of mevalonate metabolites for primary and secondary metabolism in Catharanthus roseus cell suspensions. Physiol Plant 98:803–809
Irmler S, Schroder G, St-Pierre B, Crouch NP, Hotze M, Schmidt J, Strack D, Matern U, Schroder J (2000) Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J 24:797–804
Kruczynsky A, Hill BT (2001) Vinflunine, the latest Vinca alkaloid in clinical development. A review of its preclinical anticancer properties. Crit Rev Oncol Hematol 40:159–173
Levy HR, Popjak G (1960) Studies on the biosynthesis of cholesterol. 10. Mevalonic kinase from liver. Biochem J 75:417–428
Li J, Last RL (1996) The Arabidopsis thaliana trp5 mutant has a feedback-resistant anthranilate synthase and elevated soluble tryptophan. Plant Physiol 110:51–59
Lichtenthaler HK, Schwender J, Disch A, Rhomer M (1997) Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a so far unexpected novel pathway. FEBS Lett 400:271–274
Memelink J, Verpoorte R, Kijne JW (2001) ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci 6:212–219
Menke FL, Champion A, Kijne JW, Memelink J (1999) A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO J 18:4455–4463
Mérillon J, Doireau P, Guillot A, Chénieux JC, Rideau M (1986) Indole alkaloid accumulation and tryptophane decarboxylase activity in Catharanthus roseus cells cultured in three different media. Plant Cell Rep 5:23–26
Moreno PR, Schlatmann JE, van der Heijden R, van Gulik WM, ten Hoopen HJ, Verpoorte R, Heijnen JJ (1993) Induction of ajmalicine formation and related enzyme activities in Catharanthus roseus cells: effect of inoculum density. Appl Microbiol Biotechnol 39:42–47
Morgan JA, Shanks JV (2000) Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol 79:137–145
Naudascher F, Doireau P, Guillot A, Viel C, Thiersault M (1989) Time-course studies on the use of secologanin by Catharanthus roseus cells cultured in vitro. J Plant Physiol 134:608–612
Papon N, Bremer J, Vansiri A, Andreu F, Rideau M, Crèche J (2005) Cytokinin and ethylene control indole alkaloid production at the level of the MEP/terpenoid pathways in Catharanthus roseus suspension cells. Planta Med 71:572–574
Pasquali G, Goddijn OJ, de Waal A, Verpoorte R, Schilperoort RA, Hoge JH, Memelink J (1992) Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol Biol 18:1121–1131
Pauw B, Hilliou FA, Martin VS, Chatel G, de Wolf CJ, Champion A, Pre M, van Duijn B, Kijne JW, van der Fits L, Memelink J (2004) Zinc finger proteins act as transcriptional repressors of alkaloid biosynthesis genes in Catharanthus roseus. J Biol Chem 279:52940–52948
Pennings EJM, Groen BW, Duine JA, Verpoorte R (1989) Tryptophan decarboxylase from Catharanthus roseus is a pyridoxoquinoprotein. FEBS Lett 255:97–100
Rodriguez-Concepcion M, Yalovsky S, Zik M, Fromm H, Gruissem W (1999) The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J 18:1996–2007
Rohmer M, Knani M, Simonin P, Sutter B, Sahm H (1993) Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J 295:517–524
Sacchettini JC, Poulter CD (1997) Creating isoprenoid diversity. Science 277:1788–9
Schuhr CA, Radykewicz T, Sagner S, Latzel C, Zenk MH, Arigoni D, Bacher A, Rohdich F, Eisenreich W (2003) Quantitative assessment of crosstalk between the two isoprenoid biosynthesis pathways in plants by NMR spectroscopy. Phytochem Rev 2:3–16
Seabra MC, Reiss Y, Casey PJ, Brown MS, Golstein JL (1991) Protein farnesyltransferase and geranylgeranyltransferase share a common alpha subunit. Cell 65:429–434
Sibéril Y, Doireau P, Gantet P (2001) Plant bZIP G-box binding factors. Modular structure and activation mechanisms. Eur J Biochem 268:5655–5666
Sinensky M (2000a) Functional aspects of polyisoprenoid protein substituents:roles in protein-protein interaction and trafficking. Biochim Biophys Acta 1529:203–209
Sinensky M (2000b) Recent advances in the study of prenylated proteins. Biochim Biophys Acta 1484:93–106
Stamatakis K, Cernuda-Morollon E, Hernandez-Perera O, Perez-Sala D (2002) Isoprenylation of RhoB is necessary for its degradation. A novel determinant in the complex regulation of RhoB expression by the mevalonate pathway. J Biol Chem 277:49389–49396
van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
van der Fits L, Zhang H, Menke FL, Deneka M, Memelink J (2000) A Catharanthus roseus BPF-1 homologue interacts with an elicitor-responsive region of the secondary metabolite biosynthetic gene Str and is induced by elicitor via a JA-independent signal transduction pathway. Plant Mol Biol 44:675–685
Veau B, Courtois M, Oudin A, Chénieux JC, Rideau M, Clastre M (2000) Cloning and expression of cDNAs encoding two enzymes of the MEP pathway in Catharanthus roseus. Biochim Biophys Acta 1517:159–163
Vom Endt D, Kijne JW, Memelink J (2002) Transcription factors controlling plant secondary metabolism: what regulates the regulators?. Phytochemistry 61:107–114
Whitmer S, Canel C, Hallard D, Goncalves C, Verpoorte R (1998) Influence of precursor availability on alkaloid accumulation by transgenic cell line of Catharanthus roseus. Plant Physiol 116:853–857
Whitmer S, van der Heijden R, Verpoorte R (2002) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96:193–203
Yahia A, Kevers C, Gaspar T, Chénieux JC, Rideau M, Crèche J (1998) Cytokinins and ethylene stimulate indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus by two distinct mechanisms. Plant Sci 133:9–15
Zhang FL, Casey PJ (1996) Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 65:241–269
Zenk MH, El-Shagi H, Arens H, Stöckigt J, Weiler EW, Dues B (1977) Formation of indole alkaloids serpentine and ajmalicine in cell suspension cultures of C roseus. In: Barz W, Reinhard E, Zenk MH (eds) Plant tissue culture and its biotechnological applications. Springer-Verlag, Berlin, pp 27–44
Acknowledgements
This work is supported financially by the Région Centre and the Ligue Nationale Contre le Cancer (Comité d’Indre et Loire). Authors are grateful to Dr Pratap Pati, Guru Nanak Dev University, India for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hedhili, S., Courdavault, V., Giglioli-Guivarc’h, N. et al. Regulation of the terpene moiety biosynthesis of Catharanthus roseus terpene indole alkaloids. Phytochem Rev 6, 341–351 (2007). https://doi.org/10.1007/s11101-006-9021-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-006-9021-5