Abstract
Nanomedicine is a rapidly emerging field with several breakthroughs in the therapeutic drug delivery application. The unique properties of the nanoscale delivery systems offer huge advantages to their payload such as solubilization, increased bioavailability, and improved pharmacokinetics with an overall goal of enhanced therapeutic index. Nanomedicine has the potential for integrating and enabling new therapeutic modalities. Several nanoparticle-based drug delivery systems have been granted approval for clinical use based on their outstanding clinical outcomes. Nanomedicine faces several challenges that hinder the realization of its full potential. In this review, we discuss the critical formulation- and biological-related quality features that significantly influence the performance of nanoparticulate systems in vivo. We also discuss the quality-by-design approach in the pharmaceutical manufacturing and its implementation in the nanomedicine. A deep understanding of these nanomedicine quality checkpoints and a systematic design that takes them into consideration will hopefully expedite the clinical translation process.

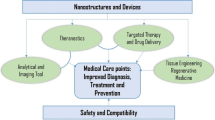
Graphical abstract




Similar content being viewed by others
Abbreviations
- ADCs:
-
Antibody-drug conjugates
- AuNPs:
-
Gold nanoparticles
- BBB:
-
Blood-brain barrier
- BxPC3:
-
Human pancreatic cancer cell line
- cGMP:
-
Current good manufacturing practice
- CNS:
-
Central nervous system
- C26:
-
Colon adenocarcinoma
- CMAs:
-
Critical material attributes
- CPPs:
-
Critical process parameters
- CQAs:
-
Critical quality attributes
- CS:
-
Control strategy
- DoE:
-
Design of experiment
- DP:
-
Drug product
- DS:
-
Design space
- EPR:
-
Enhanced permeation and retention
- FDA:
-
Food and Drug Administration
- FMEA:
-
Failure mode and effect analysis
- GSH:
-
Glutathione
- HER-2:
-
Human epidermal growth factor receptor 2
- ICH:
-
International Conference of Harmonization
- MPS:
-
Mononuclear phagocytic system
- PAT:
-
Process analytical technology
- PEG:
-
Polyethylene glycol
- PK:
-
Pharmacokinetics
- PLGA:
-
Poly (lactic-co-glycolic acid)
- QbD:
-
Quality-by-design
- QTPP:
-
Quality target product profile
- RES:
-
Reticuloendothelial system
- siRNA:
-
Small interfering RNA
- SNEDDS:
-
Self-nanoemulsifying drug delivery systems
- TAMs:
-
Tumor-associated macrophages
- TPP:
-
Target product profile
References
Webster TJ. Nanomedicine: what's in a definition? Int J Nanomedicine. 2006;1(2):115–6.
Wang N, et al. A spherical nucleic acid-based two-photon nanoprobe for RNase H activity assay in living cells and tissues. Nanoscale. 2019;11(17):8133–7.
Lewis JM, Vyas AD, Qiu Y, Messer KS, White R, Heller MJ. Integrated analysis of exosomal protein biomarkers on alternating current electrokinetic chips enables rapid detection of pancreatic cancer in patient blood. ACS Nano. 2018;12(4):3311–20.
Yasui T, Yanagida T, Ito S, Konakade Y, Takeshita D, Naganawa T, et al. Unveiling massive numbers of cancer-related urinary-microRNA candidates via nanowires. Sci Adv. 2017;3(12):e1701133.
Peng B, et al. Ultrabright fluorescent cellulose acetate nanoparticles for imaging tumors through systemic and topical applications. Mater Today. 2019;23:16–25.
Sharifi S, Seyednejad H, Laurent S, Atyabi F, Saei AA, Mahmoudi M. Superparamagnetic iron oxide nanoparticles for in vivo molecular and cellular imaging. Contrast Media Mol Imaging. 2015;10(5):329–55.
Park J, Xu M, Li F, Zhou HC. 3D long-range triplet migration in a water-stable metal–organic framework for upconversion-based ultralow-power in vivo imaging. J Am Chem Soc. 2018;140(16):5493–9.
Jiang Y, et al. Metabolizable semiconducting polymer nanoparticles for second near-infrared photoacoustic imaging. Adv Mater. 2019;31(11):1808166.
Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE. Nanoparticles in tissue engineering: applications, challenges and prospects. Int J Nanomedicine. 2018;13:5637–55.
Fathi-Achachelouei M, et al. Use of nanoparticles in tissue engineering and regenerative medicine. Front Bioeng Biotech. 2019:7(113).
Kouhi M, et al. Bredigite reinforced electrospun nanofibers for bone tissue engineering. Materials Today: Proceedings. 2019;7:449–54.
Sahoo S, et al. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A. 2010;93A(4):1539–50.
Shevach M, et al. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J Mater Chem B. 2013;1(39):5210–7.
Fleischer S, Shevach M, Feiner R, Dvir T. Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues. Nanoscale. 2014;6(16):9410–4.
You C, Li Q, Wang X, Wu P, Ho JK, Jin R, et al. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci Rep. 2017;7(1):10489.
Marsich E, Bellomo F, Turco G, Travan A, Donati I, Paoletti S. Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: preparation, characterization and biological properties. J Mater Sci Mater Med. 2013;24(7):1799–807.
Lopes-de-Campos D, Pinto RM, Lima SAC, Santos T, Sarmento B, Nunes C, et al. Delivering amoxicillin at the infection site—a rational design through lipid nanoparticles. Int J Nanomedicine. 2019;14:2781–95.
Galindo-Rodriguez SA, et al. Polymeric nanoparticles for oral delivery of drugs and vaccines: a critical evaluation of in vivo studies. 2005;22(5):419–64.
Tsai L-C, Chen CH, Lin CW, Ho YC, Mi FL. Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int J Biol Macromol. 2019;126:141–50.
Talegaonkar S, Bhattacharyya A. Potential of lipid nanoparticles (SLNs and NLCs) in enhancing oral bioavailability of drugs with poor intestinal permeability. AAPS PharmSciTech. 2019;20(3):121.
Mazumder S, Dewangan AK, Pavurala N. Enhanced dissolution of poorly soluble antiviral drugs from nanoparticles of cellulose acetate based solid dispersion matrices. Asian J Pharm Sci. 2017;12(6):532–41.
Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–23.
Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of nanoparticles. Nat Biotechnol. 2007;25(10):1165–70.
Ponnuswamy N, et al. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat Commun. 2017;8:15654.
Li S-D, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5(4):496–504.
Zhang L, et al. Targeted codelivery of an antigen and dual agonists by hybrid nanoparticles for enhanced Cancer immunotherapy. Nano Lett. 2019;19(7):4237–49.
Sun W, et al. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials. 2019;217:119264.
Xu J, et al. Quinic acid-conjugated nanoparticles enhance drug delivery to solid tumors via interactions with endothelial Selectins. Small. 2018;14(50):1803601.
Shevtsov M, et al. Granzyme B functionalized nanoparticles targeting membrane Hsp70-positive tumors for multimodal cancer theranostics. Small. 2019;15(13):1900205.
Hua, S., et al., Current Trends and Challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol, 2018. 9(790).
Yadav, H.K.S., et al., Chapter 17 - polymer-based nanomaterials for drug-delivery carriers, in nanocarriers for drug delivery, S.S. mohapatra, et al., Editors. 2019, Elsevier. p. 531–556.
Miao T, Wang J, Zeng Y, Liu G, Chen X. Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering: from bench to bedside. Advanced Science. 2018;5(4):1700513.
Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–63.
Seifu MF, Nath LK. Polymer-drug conjugates: novel carriers for cancer chemotherapy. Polymer Plast Technol Mater. 2019;58(2):158–71.
Gregoriadis G, Florence AT. Liposomes in drug delivery. Drugs. 1993;45(1):15–28.
Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–13.
Zhao Y, Huang L. Lipid nanoparticles for gene delivery. Adv Genet. 2014;88:13–36.
El-Zahaby SA, et al. Development of a novel solid self-nano-emulsifying osmotically controlled system of a centrally acting drug: preparation and in-vitro evaluation. Inventi Impact: NDDS. 2016;1:35–49.
Giner-Casares JJ, et al. Inorganic nanoparticles for biomedicine: where materials scientists meet medical research. Mater Today. 2016;19(1):19–28.
Chakravarty, R., et al., Radiolabeled inorganic nanoparticles for positron emission tomography imaging of cancer: an overview. The Quarterly Journal of Nuclear Medicine and Molecular Imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the Society of... 2017. 61(2): p. 181–204.
Nune SK, Gunda P, Thallapally PK, Lin YY, Forrest ML, Berkland CJ. Nanoparticles for biomedical imaging. Expert Opin Drug Del. 2009;6(11):1175–94.
Kosaka N, et al. Real-time optical imaging using quantum dot and related nanocrystals. Nanomedicine (London, England). 2010;5(5):765–76.
Arvizo RR, Bhattacharyya S, Kudgus RA, Giri K, Bhattacharya R, Mukherjee P. Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future. Chem Soc Rev. 2012;41(7):2943–70.
Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77(7):2325–31.
Al-Ahmady ZS, et al. Enhanced intra-liposomal metallic nanoparticle payload capacity using microfluidics assisted self-assembly. Langmuir. 2019.
Hao Y, Zhang B, Zheng C, Ji R, Ren X, Guo F, et al. The tumor-targeting core–shell structured DTX-loaded PLGA@Au nanoparticles for chemo-photothermal therapy and X-ray imaging. J Control Release. 2015;220:545–55.
Khan MM, Madni A, Torchilin V, Filipczak N, Pan J, Tahir N, et al. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019;26(1):765–72.
Haidar ZS, Hamdy RC, Tabrizian M. Protein release kinetics for core–shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials. 2008;29(9):1207–15.
Cook J. Nanoparticles approved in the United States (US) and Europe (EU) for Medical Applications. 2017 11/2019]; Available from: https://nanohybrids.net/blogs/nanoparticles/fda-ema-approved-nanoparticles.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1(1):10–29.
Desai, N., Nab technology: a drug delivery platform utilising endothelial gp60 receptor-based transport and tumour-derived SPARC for targeting drug delivery report. 2008. 2007/2008: p. 37–41.
Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl Oncol. 2009;2(2):59–64.
Chen EC, Fathi AT, Brunner AM. Reformulating acute myeloid leukemia: liposomal cytarabine and daunorubicin (CPX-351) as an emerging therapy for secondary AML. OncoTargets Ther. 2018;11:3425–34.
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–87.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioengineering Transla Med. 2019;4(3):e10143.
Kumar, G.L., FDA-approved targeted therapies in oncology, in predictive biomarkers in oncology: applications in precision medicine, S. Badve and G.L. Kumar, Editors. 2019, Springer International Publishing: Cham p 605-622.
Sengupta S. Cancer nanomedicine: lessons for immuno-oncology. Trends in Cancer. 2017;3(8):551–60.
Park K. The beginning of the end of the nanomedicine hype. J Control Release. 2019;10(305):221–2.
Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014.
Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9(2):223–43.
Liang, Y., Drug release and pharmacokinetic properties of liposomal DB-67, in Graduate school2010, University of Kentucky.
Dunne M, Corrigan I, Ramtoola Z. Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials. 2000;21(16):1659–68.
Braet F, Wisse E, Bomans P, Frederik P, Geerts W, Koster A, et al. Contribution of high-resolution correlative imaging techniques in the study of the liver sieve in three-dimensions. Microsc Res Tech. 2007;70(3):230–42.
Ballet F. Hepatic circulation: potential for therapeutic intervention. Pharmacol Ther. 1990;47(2):281–328.
Chen L-T, Weiss L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood. 1973;41(4):529–37.
Iyer AK, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17):812–8.
Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6(12):815–23.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377(Pt 1):159–69.
Guo X, et al. Multifunctional nanoplatforms for subcellular delivery of drugs in cancer therapy. Prog Mater Sci. 2020;107:100599.
Nowak M, Helgeson ME, Mitragotri S. Delivery of nanoparticles and macromolecules across the blood–brain barrier. Adv Ther. 2019;0(0):1900073.
Pei Y, Mohamed MF, Seleem MN, Yeo Y. Particle engineering for intracellular delivery of vancomycin to methicillin-resistant Staphylococcus aureus (MRSA)-infected macrophages. J Control Release. 2017;267:133–43.
Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–15.
Laine GA, et al. Polyethylene glycol nephrotoxicity secondary to prolonged high-dose intravenous Lorazepam. Ann Pharmacother. 1995;29(11):1110–4.
Rocca JD, Liu D, Lin W. Are high drug loading nanoparticles the next step forward for chemotherapy? Nanomedicine (London, England). 2012;7(3):303–5.
Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70(1–2):1–20.
Radwan MA. In vitro evaluation of Polyisobutylcyanoacrylate nanoparticles as a controlled drug carrier for theophylline. Drug Dev Ind Pharm. 1995;21(20):2371–5.
Liu L, Ma P, Wang H, Zhang C, Sun H, Wang C, et al. Immune responses to vaccines delivered by encapsulation into and/or adsorption onto cationic lipid-PLGA hybrid nanoparticles. J Control Release. 2016;225:230–9.
Kufleitner J, Wagner S, Worek F, von Briesen H, Kreuter J. Adsorption of obidoxime onto human serum albumin nanoparticles: drug loading, particle size and drug release. J Microencapsul. 2010;27(6):506–13.
O'Hagan D, Singh M, Ugozzoli M, Wild C, Barnett S, Chen M, et al. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J Virol. 2001;75(19):9037–43.
Dragojevic S, Ryu J, Raucher D. Polymer-based prodrugs: improving tumor targeting and the solubility of small molecule drugs in Cancer therapy. Molecules. 2015;20(12):19804.
Cholkar, K., et al., Chapter 1—therapeutic applications of polymeric materials, in emerging nanotechnologies for diagnostics, drug delivery and medical devices2017, Elsevier: Boston. p. 1–19.
Yoo HS, Park TG. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA–PEG block copolymer. J Control Release. 2001;70(1):63–70.
Xu, X., G.R. Shan, and P. Pan, Controlled co-delivery of hydrophilic and hydrophobic drugs from thermosensitive and crystallizable copolymer nanoparticles. J Appl Polym Sci, 2016. 133(42): p. n/a-n/a.
Tao X, et al. Effects of particle hydrophobicity, surface charge, media ph value and complexation with human serum albumin on drug release behavior of mitoxantrone-loaded pullulan nanoparticles. Nanomaterials. 2016:6(1).
Kim JO, Kabanov AV, Bronich TK. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J Control Release. 2009;138(3):197–204.
Diab R, Jaafar-Maalej C, Fessi H, Maincent P. Engineered nanoparticulate drug delivery systems: the next frontier for oral administration? AAPS J. 2012;14(4):688–702.
Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly (ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98(3):415–26.
Glavas L, Olsén P, Odelius K, Albertsson AC. Achieving micelle control through core crystallinity. Biomacromolecules. 2013;14(11):4150–6.
Liu KC, Yeo Y. Extracellular stability of nanoparticulate drug carriers. Arch Pharm Res. 2014;37(1):16–23.
Ao L, Reichel D, Hu D, Jeong H, Kim KB, Bae Y, et al. Polymer micelle formulations of proteasome inhibitor Carfilzomib for improved metabolic stability and anticancer efficacy in human multiple myeloma and lung cancer cell lines. J Pharmacol Exp Ther. 2015;355(2):168–73.
Park JE, Chun SE, Reichel D, Min JS, Lee SC, Han S, et al. Polymer micelle formulation for the proteasome inhibitor drug carfilzomib: anticancer efficacy and pharmacokinetic studies in mice. PLoS One. 2017;12(3):e0173247.
Hamaguchi T, Kato K, Yasui H, Morizane C, Ikeda M, Ueno H, et al. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br J Cancer. 2007;97(2):170–6.
Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane(®) ABI-007) in the treatment of breast cancer. Int J Nanomedicine. 2009;4:99–105.
Garcia AA, Kempf RA, Rogers M, Muggia FM. A phase II study of Doxil (liposomal doxorubicin): lack of activity in poor prognosis soft tissue sarcomas. Ann Oncol. 1998;9(10):1131–3.
Ellerhorst JA, Bedikian A, Ring S, Buzaid AC, Eton O, Legha SS. Phase II trial of doxil for patients with metastatic melanoma refractory to frontline therapy. Oncol Rep. 1999;6(5):1097–9.
Andresen TL, Jensen SS, Jørgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44(1):68–97.
Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358(2):100–6.
Jyoti A, et al. An in vitro assessment of liposomal topotecan simulating metronomic chemotherapy in combination with radiation in tumor-endothelial spheroids. Sci Rep. 2015;5:15236–6.
Haran G, et al. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta Biomembr. 1993;1151(2):201–15.
Hu J, Guo J, Xie Z, Shan D, Gerhard E, Qian G, et al. Fluorescence imaging enabled poly (lactide-co-glycolide). Acta Biomater. 2016;29:307–19.
Weinkauf DH and Paul DR. Effects of structural order on barrier properties, in barrier polymers and structures1990, American Chemical Society p 60-91.
Karavelidis V, Karavas E, Giliopoulos D, Papadimitriou S, Bikiaris D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int J Nanomedicine. 2011;6:3021–32.
Saltzman WM. Drug delivery : engineering principles for drug therapy2001, Cary, UNITED STATES: Oxford University Press USA - OSO.
Alavizadeh SH, et al. The influence of phospholipid on the physicochemical properties and anti-tumor efficacy of liposomes encapsulating cisplatin in mice bearing C26 colon carcinoma. Int J Pharm. 2014;473(1):326–33.
Aguilar, Z.P., Chapter 5—targeted drug delivery, in nanomaterials for medical applications, Z.P. Aguilar, Editor 2013, Elsevier. p. 181–234.
Lu X.-Y., et al., Chpater 7—polymer nanoparticles, in progress in molecular biology and translational science, A. Villaverde, Editor 2011, Academic Press. p. 299–323.
Rijcken Cristianne, J.F., et al., Therapeutic nanomedicine: cross linked micelles with transiently linked drugs—a versatile drug delivery system, in Eur J Nanomed 2010. p. 19.
Tao X, Zhang Q, Ling K, Chen Y, Yang W, Gao F, et al. Effect of pullulan nanoparticle surface charges on HSA complexation and drug release behavior of HSA-bound nanoparticles. PLoS One. 2012;7(11):e49304.
Taha MS, et al. Sustained delivery of Carfilzomib by tannic acid-based nanocapsules helps develop antitumor immunity. Nano Lett. 2019.
Zhang L, et al. Self-assembled lipid−polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2(8):1696–702.
Crielaard BJ, et al. Glucocorticoid-loaded core-cross-linked polymeric micelles with tailorable release kinetics for targeted therapy of rheumatoid arthritis. Angew Chem Int Ed Eng. 2012;51(29):7254–8.
Tong R, Cheng J. Paclitaxel-initiated, controlled polymerization of Lactide for the formulation of polymeric nanoparticulate delivery vehicles. Angew Chem Int Ed. 2008;47(26):4830–4.
Tsukamoto T, et al. Preparation of bromfenac-loaded liposomes modified with chitosan for ophthalmic drug delivery and evaluation of physicochemical properties and drug release profile. Asian J Pharm Sci. 2013;8(2):104–9.
Holkar CR, et al., Chapter 14—scale-up technologies for advanced nanomaterials for green energy: feasibilities and challenges, in nanomaterials for green energy, B.A. Bhanvase, et al., Editors. 2018, Elsevier. p. 433–455.
Dormont F, Rouquette M, Mahatsekake C, Gobeaux F, Peramo A, Brusini R, et al. Translation of nanomedicines from lab to industrial scale synthesis: the case of squalene-adenosine nanoparticles. J Control Release. 2019;307:302–14.
Sepulveda CA, et al. Establishing a GMP manufacturing site for nanoparticles. NSTI nanotechnology conference and trade show - NSTI nanotech 2007. Technical Proceedings. 2007;2:413–6.
Coty J-B, Vauthier C. Characterization of nanomedicines: a reflection on a field under construction needed for clinical translation success. J Control Release. 2018;275:254–68.
Galindo-Rodríguez SA, et al. Comparative scale-up of three methods for producing ibuprofen-loaded nanoparticles. Eur J Pharm Sci. 2005;25(4):357–67.
Soares S, et al. Nanomedicine: principles, properties, and regulatory issues. Front Chem. 2018;6:360.
Lundqvist M, Stigler J, Cedervall T, Berggård T, Flanagan MB, Lynch I, et al. The evolution of the protein corona around nanoparticles: a test study. ACS Nano. 2011;5(9):7503–9.
Rezaei G, Daghighi SM, Haririan I, Yousefi I, Raoufi M, Rezaee F, et al. Protein corona variation in nanoparticles revisited: a dynamic grouping strategy. Colloids Surf B: Biointerfaces. 2019;179:505–16.
Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8(7):543–57.
Chen D, Ganesh S, Wang W, Amiji M. The role of surface chemistry in serum protein corona-mediated cellular delivery and gene silencing with lipid nanoparticles. Nanoscale. 2019;11(18):8760–75.
Phogat N, et al., Chapter 11—interaction of nanoparticles with biomolecules, protein, enzymes, and its applications, in precision medicine, H.-P. Deigner and M. Kohl, Editors. 2018, Academic Press. p. 253–276.
Gunawan C, et al. Nanoparticle–protein corona complexes govern the biological fates and functions of nanoparticles. J Mater Chem B. 2014;2(15):2060–83.
Tenzer S, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8:772.
Salvati A, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137.
Lundqvist M, Augustsson C, Lilja M, Lundkvist K, Dahlbäck B, Linse S, et al. The nanoparticle protein corona formed in human blood or human blood fractions. PLoS One. 2017;12(4):e0175871.
Xiao W, Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int J Pharm. 2018;552(1–2):328–39.
Tonigold M, Simon J, Estupiñán D, Kokkinopoulou M, Reinholz J, Kintzel U, et al. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat Nanotechnol. 2018;13(9):862–9.
Safavi-Sohi R, Maghari S, Raoufi M, Jalali SA, Hajipour MJ, Ghassempour A, et al. Bypassing protein corona issue on active targeting: Zwitterionic coatings dictate specific interactions of targeting moieties and cell receptors. ACS Appl Mater Interfaces. 2016;8(35):22808–18.
Guan J, Shen Q, Zhang Z, Jiang Z, Yang Y, Lou M, et al. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun. 2018;9(1):2982.
Zhang Z, Guan J, Jiang Z, Yang Y, Liu J, Hua W, et al. Brain-targeted drug delivery by manipulating protein corona functions. Nat Commun. 2019;10(1):3561.
Oh JY, Kim HS, Palanikumar L, Go EM, Jana B, Park SA, et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat Commun. 2018;9(1):4548.
Corbo C, Molinaro R, Tabatabaei M, Farokhzad OC, Mahmoudi M. Personalized protein corona on nanoparticles and its clinical implications. Biomater Sci. 2017;5(3):378–87.
Shahabi S, et al. Modulation of silica nanoparticle uptake into human osteoblast cells by variation of the ratio of amino and Sulfonate surface groups: effects of serum. ACS Appl Mater Interfaces. 2015;7(25):13821–33.
Pillai GJ, Greeshma MM, Menon D. Impact of poly (lactic-co-glycolic acid) nanoparticle surface charge on protein, cellular and haematological interactions. Colloids Surf B: Biointerfaces. 2015;136:1058–66.
Elci SG, Jiang Y, Yan B, Kim ST, Saha K, Moyano DF, et al. Surface charge controls the suborgan biodistributions of gold nanoparticles. ACS Nano. 2016;10(5):5536–42.
Fromen CA, Rahhal TB, Robbins GR, Kai MP, Shen TW, Luft JC, et al. Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells. Nanomedicine. 2016;12(3):677–87.
Quan X, Peng C, Zhao D, Li L, Fan J, Zhou J. Molecular understanding of the penetration of functionalized gold nanoparticles into asymmetric membranes. Langmuir. 2017;33(1):361–71.
Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012;7:5577–91.
Shao XR, et al. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015;48(4):465–74.
Sohail MF, et al. Advancements in the oral delivery of Docetaxel: challenges, current state-of-the-art and future trends. Int J Nanomedicine. 2018;13:3145–61.
Poovi G, Damodharan N. Lipid nanoparticles: a challenging approach for oral delivery of BCS class-II drugs. Future J Pharm Sci. 2018;4(2):191–205.
Narayanan D, Pillai GJ, Nair SV, Menon D. Effect of formulation parameters on pharmacokinetics, pharmacodynamics, and safety of diclofenac nanomedicine. Drug Deliv Transl Res. 2019;9(5):867–78.
Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268–86.
Zeeshan M. et al. A boon to the brain-targeted drug delivery. Pharmaceutical formulation design—recent practices: Nanopharmaceuticals; 2019.
Padmakumar S, Parayath N, Leslie F, Nair SV, Menon D, Amiji MM. Intraperitoneal chemotherapy for ovarian cancer using sustained-release implantable devices. Expert Opin Drug Del. 2018;15(5):481–94.
Padmakumar S, Paul-Prasanth B, Pavithran K, Vijaykumar DK, Rajanbabu A, Sivanarayanan TB, et al. Long-term drug delivery using implantable electrospun woven polymeric nanotextiles. Nanomedicine. 2019;15(1):274–84.
Rai, P. and S.A. Morris, Nanotheranostics for cancer applications2019, Springer.
Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley interdisciplinary reviews. Nanomed Nanobi. 2013;5(3):205–18.
Raju G, Katiyar N, Vadukumpully S, Shankarappa SA. Penetration of gold nanoparticles across the stratum corneum layer of thick-skin. J Dermatol Sci. 2018;89(2):146–54.
Palmer, B.C. and L.A. DeLouise, Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules, 2016. 21(12).
Cevc G. Lipid vesicles and other colloids as drug carriers on the skin. Adv Drug Deliv Rev. 2004;56(5):675–711.
Bhatia A, et al. Tamoxifen-loaded novel liposomal formulations: evaluation of anticancer activity on DMBA-TPA induced mouse skin carcinogenesis. J Drug Target. 2012;20(6):544–50.
Krause P, et al. Pharmacokinetics of intravesical versus oral oxybutynin in healthy adults: results of an open label, randomized, prospective clinical study. J Urol. 2013;190(5):1791–7.
Zacchè MM, Srikrishna S, Cardozo L. Novel targeted bladder drug-delivery systems: a review. Res Rep Urol. 2015;7:169–78.
Kates M, Date A, Yoshida T, Afzal U, Kanvinde P, Babu T, et al. Preclinical evaluation of Intravesical cisplatin nanoparticles for non-muscle-invasive bladder cancer. Clin Cancer Res. 2017;23(21):6592–601.
Lu Z, Yeh TK, Tsai M, Au JL, Wientjes MG. Paclitaxel-loaded gelatin nanoparticles for intravesical bladder cancer therapy. Clin Cancer Res. 2004;10(22):7677–84.
van Vlerken LE, Amiji MM. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin Drug Del. 2006;3(2):205–16.
Ojha T, et al. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv Drug Deliv Rev. 2017;119:44–60.
Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6.
Rosenblum D, et al. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410.
Rahman AM, Yusuf SW, Ewer MS. Anthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulation. Int J Nanomedicine. 2007;2(4):567–83.
Li C, et al. Dual-ligand modification of PEGylated liposomes used for targeted doxorubicin delivery to enhance anticancer efficacy. AAPS Pharm Sci Tech. 2019;20(5):019–1385.
Agrahari V, Agrahari V. Facilitating the translation of nanomedicines to a clinical product: challenges and opportunities. Drug Discov Today. 2018;23(5):974–91.
Zhou L, Wang H, Li Y. Stimuli-responsive nanomedicines for overcoming cancer multidrug resistance. Theranostics. 2018;8(4):1059–74.
Wang S, Huang P, Chen X. Stimuli-responsive programmed specific targeting in nanomedicine. ACS Nano. 2016;10(3):2991–4.
Shankarappa SA, Koyakutty M, Nair SV. Efficacy versus toxicity—the Ying and Yang in translating nanomedicines. Nanomater Nanotechno. 2014;4:23.
Dobrovolskaia MA. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: challenges, considerations and strategy. J Control Release. 2015;220(Pt B):571–83.
Arima A, Tsutsui M, Harlisa IH, Yoshida T, Tanaka M, Yokota K, et al. Selective detections of single-viruses using solid-state nanopores. Sci Rep. 2018;8(1):16305.
Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–95.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51.
Caracciolo G, Farokhzad OC, Mahmoudi M. Biological identity of nanoparticles in vivo: clinical implications of the protein corona. Trends Biotechnol. 2017;35(3):257–64.
Bastogne T. Quality-by-design of nanopharmaceuticals—a state of the art. Nanomedicine. 2017;13(7):2151–7.
Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: I. formulation, processing design and risk assessment. Int J Pharm. 2011;419(1–2):52–9.
Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: II. Screening of critical variables, and establishment of design space at laboratory scale. Int J Pharm. 2012;423(2):543–53.
Xu X, Costa AP, Khan MA, Burgess DJ. Application of quality by design to formulation and processing of protein liposomes. Int J Pharm. 2012;434(1–2):349–59.
Pallagi E, et al. Application of the QbD-based approach in the early development of liposomes for nasal administration. Int J Pharm. 2019;562:11–22.
Amasya G, Aksu B, Badilli U, Onay-Besikci A, Tarimci N. QbD guided early pharmaceutical development study: production of lipid nanoparticles by high pressure homogenization for skin cancer treatment. Int J Pharm. 2019;563:110–21.
Iurian S, Bogdan C, Tomuță I, Szabó-Révész P, Chvatal A, Leucuța SE, et al. Development of oral lyophilisates containing meloxicam nanocrystals using QbD approach. Eur J Pharm Sci. 2017;104:356–65.
Kumar S, Gokhale R, Burgess DJ. Quality by design approach to spray drying processing of crystalline nanosuspensions. Int J Pharm. 2014;464(1–2):234–42.
Narayan R, Pednekar A, Bhuyan D, Gowda C, Koteshwara KB, Nayak UY. A top-down technique to improve the solubility and bioavailability of aceclofenac: in vitro and in vivo studies. Int J Nanomedicine. 2017;12:4921–35.
Shirsat AE, Chitlange SS. Application of quality by design approach to optimize process and formulation parameters of rizatriptan loaded chitosan nanoparticles. J Adv Pharm Technol Res. 2015;6(3):88–96.
Park SJ, et al. Quality by design: screening of critical variables and formulation optimization of Eudragit E nanoparticles containing dutasteride. Arch Pharm Res. 2013;36(5):593–601.
Troiano G, Nolan J, Parsons D, van Geen Hoven C, Zale S. A quality by design approach to developing and manufacturing polymeric nanoparticle drug products. AAPS J. 2016;18(6):1354–65.
Cun D, Jensen DK, Maltesen MJ, Bunker M, Whiteside P, Scurr D, et al. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: quality by design optimization and characterization. Eur J Pharm Biopharm. 2011;77(1):26–35.
Kola Srinivas NS, et al. A quality by design approach on polymeric nanocarrier delivery of gefitinib: formulation, in vitro, and in vivo characterization. Int J Nanomedicine. 2017;12:15–28.
Medarevic D, et al. Optimization of formulation and process parameters for the production of carvedilol nanosuspension by wet media milling. Int J Pharm. 2018;540(1–2):150–61.
Rawal M, Singh A, Amiji MM. Quality-by-design concepts to improve nanotechnology-based drug development. Pharm Res. 2019;36(11):153.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taha, M., Padmakumar, S., Singh, A. et al. Critical quality attributes in the development of therapeutic nanomedicines toward clinical translation. Drug Deliv. and Transl. Res. 10, 766–790 (2020). https://doi.org/10.1007/s13346-020-00744-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00744-1