ABSTRACT
Purpose
The advent of cocrystals has demonstrated a growing need for efficient and comprehensive coformer screening in search of better development forms, including salt forms. Here, we investigated a coformer screening system for salts and cocrystals based on binary phase diagrams using thermal analysis and examined the effectiveness of the method.
Methods
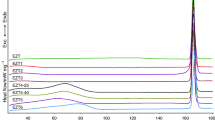
Indomethacin and tenoxicam were used as models of active pharmaceutical ingredients (APIs). Physical mixtures of an API and 42 kinds of coformers were analyzed using Differential Scanning Calorimetry (DSC) and X-ray DSC. We also conducted coformer screening using a conventional slurry method and compared these results with those from the thermal analysis method and previous studies.
Results
Compared with the slurry method, the thermal analysis method was a high-performance screening system, particularly for APIs with low solubility and/or propensity to form solvates. However, this method faced hurdles for screening coformers combined with an API in the presence of kinetic hindrance for salt or cocrystal formation during heating or if there is degradation near the metastable eutectic temperature.
Conclusions
The thermal analysis and slurry methods are considered complementary to each other for coformer screening. Feasibility of the thermal analysis method in drug discovery practice is ensured given its small scale and high throughput.








Similar content being viewed by others
REFERENCES
Aakeroy CB, Forbes S, Desper J. Using cocrystals to systematically modulate aqueous solubility and melting behavior of an anticancer drug. J Am Chem Soc. 2009;131(47):17048–9.
Remenar JF, Morissette SL, Peterson ML, Moulton B, MacPhee JM, Guzman HR, et al. Crystal engineering of novel cocrystals of a triazole drug with 1,4-dicarboxylic acids. J Am Chem Soc. 2003;125(28):8456–7.
Good DJ, Rodriguez-Hornedo N. Solubility advantage of pharmaceutical cocrystals. Cryst Growth Des. 2009;9(5):2252–64.
Trask AV, Motherwell WDS, Jones W. Physical stability enhancement of theophylline via cocrystallization. Int J Pharm. 2006;320(1–2):114–23.
Trask AV, Motherwell WDS, Jones W. Pharmaceutical cocrystallization: engineering a remedy for caffeine hydration. Cryst Growth Des. 2005;5(3):1013–21.
Karki S, Friscic T, Fabian L, Laity PR, Day GM, Jones W. Improving mechanical properties of crystalline solids by cocrystal formation: new compressible forms of paracetamol. Adv Mater. 2009;21(38–39):3905–9.
Sun CC, Hou H. Improving mechanical properties of caffeine and methyl gallate crystals by cocrystallization. Cryst Growth Des. 2008;8(5):1575–9.
McNamara DP, Childs SL, Giordano J, Iarriccio A, Cassidy J, Shet MS, et al. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm Res. 2006;23(8):1888–97.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Antidiabetic effects of SGLT2-selective inhibitor ipragliflozin in streptozotocin-nicotinamide-induced mildly diabetic mice. J Pharmacol Sci. 2012;120(1):36–44.
Mascitti V, Thuma BA, Smith AC, Robinson RP, Brandt T, Kalgutkar AS, et al. On the importance of synthetic organic chemistry in drug discovery: reflections on the discovery of antidiabetic agent ertugliflozin. MedChemComm. 2013;4(1):101–11.
Jones W, Motherwell S, Trask AV. Pharmaceutical cocrystals: an emerging approach to physical property enhancement. MRS Bull. 2006;31(11):875–9.
Stahly GP. Diversity in single- and multiple-component crystals. The search for and prevalence of polymorphs and cocrystals. Cryst Growth Des. 2007;7(6):1007–26.
Zhang GGZ, Henry RF, Borchardt TB, Lou XC. Efficient co-crystal screening using solution-mediated phase transformation. J Pharm Sci-Us. 2007;96(5):990–5.
Takata N, Shiraki K, Takano R, Hayashi Y, Terada K. Cocrystal screening of stanolone and mestanolone using slurry crystallization. Cryst Growth Des. 2008;8(8):3032–7.
Kojima T, Tsutsumi S, Yamamoto K, Ikeda Y, Moriwaki T. High-throughput cocrystal slurry screening by use of in situ Raman microscopy and multi-well plate. Int J Pharm. 2010;399(1–2):52–9.
Friscic T, Childs SL, Rizvi SAA, Jones W. The role of solvent in mechanochemical and sonochemical cocrystal formation: a solubility-based approach for predicting cocrystallisation outcome. CrystEngComm. 2009;11(3):418–26.
Weyna DR, Shattock T, Vishweshwar P, Zaworotko MJ. Synthesis and structural characterization of cocrystals and pharmaceutical cocrystals: mechanochemistry vs slow evaporation from solution. Cryst Growth Des. 2009;9(2):1106–23.
Padrela L, Rodrigues MA, Velaga SP, Fernandes AC, Matos HA, de Azevedo EG. Screening for pharmaceutical cocrystals using the supercritical fluid enhanced atomization process. J Supercrit Fluids. 2010;53(1–3):156–64.
Li ZB, Yang BS, Jiang M, Eriksson M, Spinelli E, Yee N, et al. A practical solid form screen approach to identify a pharmaceutical glutaric acid cocrystal for development. Org Process Res Dev. 2009;13(6):1307–14.
Karki S, Friscic T, Jones W, Motherwell WDS. Screening for pharmaceutical cocrystal hydrates via neat and liquid-assisted grinding. Mol Pharm. 2007;4(3):347–54.
Bysouth SR, Bis JA, Igo D. Cocrystallization via planetary milling: enhancing throughput of solid-state screening methods. Int J Pharm. 2011;411(1–2):169–71.
Patel JR, Carlton RA, Needham TE, Chichester CO, Vogt FG. Preparation, structural analysis, and properties of tenoxicam cocrystals. Int J Pharm. 2012;436(1–2):685–706.
Berry DJ, Seaton CC, Clegg W, Harrington RW, Coles SJ, Horton PN, et al. Applying hot-stage microscopy to co-crystal screening: a study of nicotinamide with seven active pharmaceutical ingredients. Cryst Growth Des. 2008;8(5):1697–712.
Yamashita H, Hirakura Y, Yuda M, Teramura T, Terada K. Detection of cocrystal formation based on binary phase diagrams using thermal analysis. Pharm Res. 2013;30(1):70–80.
Cooke CL, Davey RJ, Black S, Muryn C, Pritchard RG. Binary and ternary phase diagrams as routes to salt discovery ephedrine and pimelic acid. Cryst Growth Des. 2010;10(12):5270–8.
Alleso M, Velaga S, Alhalaweh A, Cornett C, Rasmussen MA, van den Berg F, et al. Near-infrared spectroscopy for cocrystal screening. A comparative study with Raman spectroscopy. Anal Chem. 2008;80(20):7755–64.
Basavoju S, Bostrom D, Velaga SP. Indomethacin-saccharin cocrystal: design, synthesis and preliminary pharmaceutical characterization. Pharm Res. 2008;25(3):530–41.
Umeda Y, Fukami T, Furuishi T, Suzuki T, Tanjoh K, Tomono K. Characterization of multicomponent crystal formed between indomethacin and lidocaine. Drug Dev Ind Pharm. 2009;35(7):843–51.
Cantera RG, Leza MG, Bachiller CM. Solid phases of tenoxicam. J Pharm Sci-Us. 2002;91(10):2240–51.
Wouters J. Pharmaceutical salts and co-crystals. Cambridge: The Royal Society of Chemistry; 2012.
Database of Select Committee on GRAS Substances (SCOGS) Reviews. Available from: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm084104.htm.
Stahl PH, Wermuth, Camille G. Handbook of pharmaceutical salts. 2nd ed. Weinheim: WILEY-VCH Verlag GmbH; 2011.
Nygren CL, Wilson CC, Turner JFC. Electron and nuclear positions in the short hydrogen bond in urotropine-N-oxide-formic acid. J Phys Chem A. 2005;109(9):1911–9.
Aakeroy CB, Fasulo ME, Desper J. Cocrystal or salt: does it really matter? Mol Pharm. 2007;4(3):317–22.
Lu E, Rodriguez-Hornedo N, Suryanarayanan R. A rapid thermal method for cocrystal screening. Crystengcomm. 2008;10(6):665–8.
ACKNOWLEDGMENTS AND DISCLOSURES
We thank Mayuko Mirun for assisting with the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, H., Hirakura, Y., Yuda, M. et al. Coformer Screening Using Thermal Analysis Based on Binary Phase Diagrams. Pharm Res 31, 1946–1957 (2014). https://doi.org/10.1007/s11095-014-1296-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1296-4