ABSTRACT
Purpose
Although a number of studies have reported that cocrystals can form by heating a physical mixture of two components, details surrounding heat-induced cocrystal formation remain unclear. Here, we attempted to clarify the thermal behavior of a physical mixture and cocrystal formation in reference to a binary phase diagram.
Methods
Physical mixtures prepared using an agate mortar were heated at rates of 2, 5, 10, and 30°C/min using differential scanning calorimetry (DSC). Some mixtures were further analyzed using X-ray DSC and polarization microscopy.
Results
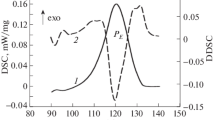
When a physical mixture consisting of two components which was capable of cocrystal formation was heated using DSC, an exothermic peak associated with cocrystal formation was detected immediately after an endothermic peak. In some combinations, several endothermic peaks were detected and associated with metastable eutectic melting, eutectic melting, and cocrystal melting. In contrast, when a physical mixture of two components which is incapable of cocrystal formation was heated using DSC, only a single endothermic peak associated with eutectic melting was detected.
Conclusion
These experimental observations demonstrated how the thermal events were attributed to phase transitions occurring in a binary mixture and clarified the relationship between exothermic peaks and cocrystal formation.














Similar content being viewed by others
REFERENCES
Jones W, Motherwell S, Trask AV. Pharmaceutical cocrystals: an emerging approach to physical property enhancement. MRS Bull. 2006;31(11):875–9.
Bhogala BR, Nangia A. Ternary and quaternary co-crystals of 1,3-cis,5-cis-cyclohexanetricarboxylic acid and 4,4′-bipyridines. New J Chem. 2008;32(5):800–7.
Stahly GP. Diversity in single- and multiple-component crystals. The search for and prevalence of polymorphs and cocrystals. Cryst Growth Des. 2007;7(6):1007–26.
Aakeroy CB, Forbes S, Desper J. Using cocrystals to systematically modulate aqueous solubility and melting behavior of an anticancer drug. J Am Chem Soc. 2009;131(47):17048–9.
Remenar JF, Morissette SL, Peterson ML, Moulton B, MacPhee JM, Guzman HR, et al. Crystal engineering of novel cocrystals of a triazole drug with 1,4-dicarboxylic acids. J Am Chem Soc. 2003;125(28):8456–7.
Good DJ, Rodriguez-Hornedo N. Solubility advantage of pharmaceutical cocrystals. Cryst Growth Des. 2009;9(5):2252–64.
Trask AV, Motherwell WDS, Jones W. Physical stability enhancement of theophylline via cocrystallization. Int J Pharm. 2006;320(1–2):114–23.
Trask AV, Motherwell WDS, Jones W. Pharmaceutical cocrystallization: engineering a remedy for caffeine hydration. Cryst Growth Des. 2005;5(3):101–21.
Karki S, Friscic T, Fabian L, Laity PR, Day GM, Jones W. Improving mechanical properties of crystalline solids by cocrystal formation: new compressible forms of paracetamol. Adv Mater. 2009;21:3905–9.
Sun CC, Hou H. Improving mechanical properties of caffeine and methyl gallate crystals by cocrystallization. Cryst Growth Des. 2008;8(5):1575–9.
McNamara DP, Childs SL, Giordano J, Iarriccio A, Cassidy J, Shet MS, et al. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm Res. 2006;23(8):1888–97.
Hickey MB, Peterson ML, Scoppettuolo LA, Morrisette SL, Vetter A, Guzman H, et al. Performance comparison of a co-crystal of carbamazepine with marketed product. Eur J Pharm Biopharm. 2007;67(1):112–9.
Chadwick K, Davey RJ, Dent G, Pritchard RG, Hunter CA, Musumeci D. Cocrystallization: a solution chemistry perspective and the case of benzophenone and diphenylamine. Cryst Growth Des. 2009;9(4):1990–9.
Jayasankar A, Reddy LS, Bethune SJ, Rodriguez-Hornedo N. Role of cocrystal and solution chemistry on the formation and stability of cocrystals with different stoichiometry. Cryst Growth Des. 2009;9(2):889–97.
Takata N, Shiraki K, Takano R, Hayashi Y, Terada K. Cocrystal screening of stanolone and mestanolone using slurry crystallization. Cryst Growth Des. 2008;8(8):3032–7.
Zhang GGZ, Henry RF, Borchardt TB, Lou XC. Efficient co-crystal screening using solution-mediated phase transformation. J Pharm Sci-Us. 2007;96(5):990–5.
Weyna DR, Shattock T, Vishweshwar P, Zaworotko MJ. Synthesis and structural characterization of cocrystals and pharmaceutical cocrystals: mechanochemistry vs slow evaporation from solution. Cryst Growth Des. 2009;9(2):1106–23.
Basavoju S, Bostrom D, Velaga SP. Indomethacin-saccharin cocrystal: design, synthesis and preliminary pharmaceutical characterization. Pharm Res. 2008;25(3):530–41.
Trask AV, van de Streek J, Motherwell WDS, Jones W. Achieving polymorphic and stoichiometric diversity in cocrystal formation: importance of solid-state grinding, powder X-ray structure determination, and seeding. Cryst Growth Des. 2005;5(6):2233–41.
Shan N, Toda F, Jones W. Mechanochemistry and co-crystal formation: effect of solvent on reaction kinetics. Chem Commun. 2002;20:2372–3.
Trask AV, Jones W. Crystal engineering of organic cocrystals by the solid-state grinding approach. Org Solid State React. 2005;254:41–70.
Aher S, Dhumal R, Mahadik K, Paradkar A, York P. Ultrasound assisted cocrystallization from solution (USSC) containing a non-congruently soluble cocrystal component pair: caffeine/maleic acid. Eur J Pharm Sci. 2010;41(5):597–602.
Chadwick K, Davey R, Cross W. How does grinding produce co-crystals? Insights from the case of benzophenone and diphenylamine. CrystEngComm. 2007;9(9):732–4.
Berry DJ, Seaton CC, Clegg W, Harrington RW, Coles SJ, Horton PN, et al. Applying hot-stage microscopy to co-crystal screening: a study of nicotinamide with seven active pharmaceutical ingredients. Cryst Growth Des. 2008;8(5):1697–712.
Dhumal RS, Kelly AL, York P, Coates PD, Paradkar A. Cocrystalization and simultaneous agglomeration using hot melt extrusion. Pharm Res. 2010;27(12):2725–33.
Castellan GW. Physical chemistry. 3rd ed. San Francisco: Benjamin Cummings Pub. Co.; 1983.
Singh NB, Das SS, Gupta P, Dwivedi MK. Phase equillibria and solidification behaviour in the vanillin-p-anisidine system. J Cryst Growth. 2008;311(1):118–22.
Dwivedi Y, Kant S, Rai SB, Rai RN. Synthesis, physicochemical and optical characterization of novel fluorescing complex: o-phenylenediamine-benzoin. J Fluoresc. 2011;21(3):1255–63.
Rai US, George S. Thermochemical studies on the eutectics and addition-compounds in the binary-systems of benzidine with P-nitrophenol, M-aminophenol and resorcinol. Thermochim Acta. 1994;243(1):17–25.
Crowley KJ, Forbes RT, York P, Nyqvist H, Camber O. Oleate salt formation and mesomorphic behavior in the propranolol oleic acid binary system. J Pharm Sci-Us. 1999;88(6):586–91.
Winfield AJ, Saidan SHA. Compound formation in phenobarbitone-urea systems. Int J Pharm. 1981;8:211–6.
Grant DW, Jacobson H, Fairbrother JE, Patel CG. Phases, in the paracetamol-phenazone system. Int J Pharm. 1980;5:109–16.
Lu E, Rodriguez-Hornedo N, Suryanarayanan R. A rapid thermal method for cocrystal screening. Cryst Eng Comm. 2008;10(6):665–8.
Sangster J. Phase diagrams and thermodynamic properties of binary systems of drugs. J Phys Chem Ref Data. 1999;28(4):889–930.
Bhatt PM, Ravindra NV, Banerjee R, Desiraju GR. Saccharin as a salt former. Enhanced solubilities of saccharinates of active pharmaceutical ingredients. Chem Commun. 2005;28(8):1073–5.
Childs SL, Rodriguez-Hornedo N, Reddy LS, Jayasankar A, Maheshwari C, McCausland L, et al. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. Cryst Eng Comm. 2008;10(7):856–64.
Fleischman SG, Kuduva SS, McMahon JA, Moulton B, Walsh RDB, Rodriguez-Hornedo N, et al. Crystal engineering of the composition of pharmaceutical phases: multiple-component crystalline solids involving carbamazepine. Cryst Growth Des. 2003;3(6):909–19.
Jayasankar A, Somwangthanaroj A, Shao ZJ, Rodriguez-Hornedo N. Cocrystal formation during cogrinding and storage is mediated by amorphous phase. Pharm Res. 2006;23(10):2381–92.
ACKNOWLEDGMENTS AND DISCLOSURES
We thank Toshikazu Adachi for supporting our experiments and Mayuko Mirun for assisting in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, H., Hirakura, Y., Yuda, M. et al. Detection of Cocrystal Formation Based on Binary Phase Diagrams Using Thermal Analysis. Pharm Res 30, 70–80 (2013). https://doi.org/10.1007/s11095-012-0850-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0850-1