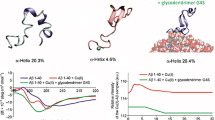
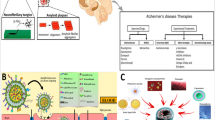
Abstract
Purpose
To develop chelating ligand-bound nanoliposomes (NLPs) for the prevention and reversal of β-Amyloid (Aβ) aggregation associated with promoting neurotoxicity in Alzheimer disease (AD).
Methods
Four different chelating ligands (CuAc, EDTA, histidine and ZnAc) were surface-engineered onto NLPs using either covalent or non-covalent conjugation. Successful conjugation of chelating ligands onto the surface of NLPs was confirmed by characterization studies: SEM, TEM and FTIR analysis. Chelation energetics of EDTA with Cu(II)/Zn(II)-Aβ(10-21) and nanoformation of emulsified polymers were computed and corroborated with experimental and analytical data using chemometric molecular modeling.
Results
The modified NLPs produced were spherical in shape, 127–178 nm in size, with polydispersity index from 0.217–0.920 and zeta potential range of −9.59 to −37.3 mV. Conjugation efficiencies were 30–76 %, which confirmed that chelating ligands were attached to the NLP surface.
Conclusions
In vitro and ex vivo results elucidated the effectiveness of chelating ligand-bound NLPs for prevention of CuAβ(1-42) or ZnAβ(1-42) aggregate buildup associated with neurotoxicity in PC12 neuronal cells, as well as promotion of intracellular uptake in the presence of Cu(II) or Zn(II) metal ions.











Similar content being viewed by others
REFERENCES
Cummings JH. Alzheimer’s disease. N Engl J Med. 2004;351:56–67.
Brookmeyer R, Evans DA, Hebert L, et al. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimer’s & Dementia. 2011;7:61–73.
Iversen LL, Mortishire-Smith RJ, Pollack SL, et al. The toxicity in vitro of beta-amyloid protein. Biochem J. 1995;311(1):1–16.
Marino T, Russo N, Toscano N, et al. On the metal ion (Zn(II), Cu (II)) coordination with beta-amyloid peptide: DFT computational study. Interdiscipl Sci. 2010;2:57–69.
De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113(11):1857–70.
Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and Aβ toxicity: from top to bottom. Nat Rev Neurosci. 2001;2(8):595–8.
Citron M. Beta-secretase as a target for the treatment of Alzheimer’s disease. J Neurosci Res. 2002;70:373–9.
De Felice FG, Vieira MN, Saraiva LM, et al. Targeting the neurotoxic species in Alzheimer’s disease: inhibitors of Aβ oligomerization. FASEB J. 2004;18:1366–72.
Lublin AL, Gandy S. Amyloid β oligemers: possible roles as key neurotoxicin in Alzheimer’s disease. Mt Sinai J Med. 2010;77(43):43–9.
Bush AI, Tanzi RE. Therapeutics for Alzheimers disease based on the metal hypothesis. Neurotherapeutics. 2008;5(3):421–32.
Lovell MA, Robertson JD, Teesdale WJ, et al. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci. 1998;158(1):47–52.
Dong J, Atwood CS, Anderson VE, et al. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochem J. 2003;42:2768–73.
Duce JA, Bush AI. Biological metals and Alzheimer’s disease: implications for therapeutics and diagnostics. Prog Neurobiol. 2010;92:1–18.
Zatta P, Drago D, Bolognin S, et al. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol Sci. 2009;30(7):346–55.
Opazo C. Transition metals and Alzheimer’s disease. Rev Esp Geriatr Gerontol. 2005;40(6):365–70.
Bush AI. Metal complexing agents as therapies for Alzheimer’s disease. Neurobiol Aging. 2002;23:1031–8.
Conway M, Holoman S, Jones L, et al. Selecting and using chelating agents. J Chem Eng. 1999;106(3):86–90.
Liu G, Men P, Kudo W, et al. Nanoparticle-chelator conjugates as inhibitors of Amyloid-β aggregation and neurotoxicity: a novel therapeutic approach for Alzheimer Disease. Neurosci Lett. 2009;455(3):187–90.
Seely DMR, Wu P, Mills EJ. EDTA chelation therapy for cardiovascular disease: a systematic review. BMC Cardiovasc. 2005;5(32):1–6.
Nair NG, Perry G, Smith MA, et al. NMR studies of zinc, copper, and iron binding to histidine, the principal metal ion complexing site of amyloid-β peptide. J Alzheimer’s Dis. 2010;20(1):57–66.
Chikha GG, Li WM, Schutze-Redelmeier MP, et al. Attaching histidine-tagged peptides and proteins to lipid-based carriers through use of metal-ion-chelating lipids. Biochim Biophys Acta. 2002;1567(1–2):204–12.
Marcellini M, Di Ciommo V, Callea F, et al. Treatment of Wilson’s disease with zinc from the time of diagnosis in pediatric patients: a single-hospital, 10-year follow-up study. J Lab Clin Med. 2005;145(3):139–43.
Shimizu N, Yamaguchi Y, Aoki T. Treatment and management of Wilson’s disease. Pediatr Int. 1999;4(4):419–22.
Squitti R, Zito G. Anti-copper therapies in Alzheimer’s disease: new concepts. Recent Pat CNS Drug Discov. 2009;4:209–19.
Popovic N, Brundin P. Therapeutic potential of controlled drug delivery systems in neurodegenerative diseases. Int J Pharm. 2006;314(2):120–6.
Cui Z, Lockman PR, Atwood CS, et al. Novel d-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer’s and other CNS diseases. Eur J Pharm Biopharm. 2005;59:263–72.
Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2:99–107.
Liu GP, Men P, Harris PL, et al. Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci Lett. 2006;406:189–93.
Atyabi F, Farkhondehfa A, Esmaeili F, et al. Preparation of pegylated nano-liposomal formulation containing SN-38: in vitro characterization and in vivo biodistribution in mice. Acta Pharm. 2009;59:133–44.
Modi G, Pillay V, Choonara YE. Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann N Y Acad Sci. 2010;1184:154–72.
Kizelsztein P, Ovadia H, Garbuzenko O, et al. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:20–5.
Yigit MV, Mishra A, Tong R, et al. Inorganic mercury detection and controlled release of chelating agents from ion-responsive liposomes. Chem Biol. 2009;16:937–42.
Phachonpai W, Wattanathorn J, Muchimapura S, et al. Neuroprotective effect of quercetin encapsulated liposomes: a novel therapeutic strategy against Alzheimer’s disease. Am J Appl Sci. 2010;7(4):480–5.
Ying X, Wen H, Lu WL, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Contr Release. 2010;141:183–92.
Kamidate T, Hashimoto Y, Tani H, et al. Uptake of transition metal ions using liposome containing dicetylphosphate as a ligand. Anal Sci. 2002;18:273–6.
Suzuki R, Takizawa T, Negishi Y, et al. Gene delivery by combination of novel liposmal bubbles with perfluoropropane and ultrasound. J Contr Release. 2007;117:130–6.
Zhua J, Xue J, Guo Z, et al. Vesicle size and stability of biomimetic liposomes from 3′-Sulfo-Lewis a (SuLea)-containing glycolipids. Colloids Surf B Biointerfaces. 2007;58(2):242–9.
Verma DD, Verma S, Blume G, et al. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258:141–51.
Yagi N, Ogawa Y, Kodaka M, et al. Preparation of functional liposomes with peptide ligands and their binding to cell membranes. Lipids. 2000;35:673–9.
Janssen AP, Schiffelers RM, ten Hagen TL, et al. Peptide-targeted PEG-liposome in anti-angiogenic therapy. Int J Pharm Pharm Sci. 2003;254(1):55–8.
Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. PNAS. 1973;73:2424–8.
Zhang LW, Yang J, Barron AR, et al. Endocytic mechanisms and toxicity of a functionalized fullerene in human cells. Toxicol Lett. 2009;191:149–57.
Kumar P, Pillay V, Choonara YE, et al. In silico theoretical molecular modeling for Alzheimer’s disease: the nicotine-curcumin paradigm in neuroprotection and neurotherapy. Int J Mol Sci. 2011;12:694–724.
Weers JG, Scheuing DR. Characterization of viscoelastic surfactant mixtures, I: fourier transform infrared spectroscopic studies. Colloids Surf B: Biointerfaces. 1991;1(55):41–56.
Strozyk D, Launer LJ, Adlard PA, et al. Zinc and copper modulate Alzheimer Aβ levels in human cerebrospinal fluid. Neurobiol Aging. 2009;30(7):1069–77.
Choonara YE, Pillay V, Ndesendo VMK, et al. Polymeric emulsion and crosslink-mediated synthesis of super-stable nanoparticles as sustained-release anti-tuberculosis drug carriers. Colloids Surf B: Biointerfaces. 2011;87:243–54.
Lozano MM, Longo ML. Microbubbles coated with disaturated lipids and DSPE-PEG2000: phase behavior, collapse transitions, and permeability. Langmuir. 2009;25:3705–12.
Morgan DM, Dong JJ, Jacob J, et al. Metal switch for amyloid formation: insight into the structure of the nucleus. J Am Chem Soc. 2002;124:12644–5.
Daxiong H, Haiyan W, Yang Y. Molecular modeling of zinc and copper binding with Alzheimer’s amyloid b-peptide. Biometals. 2008;21:189–96.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mufamadi, M.S., Choonara, Y.E., Kumar, P. et al. Surface-Engineered Nanoliposomes by Chelating Ligands for Modulating the Neurotoxicity Associated with β-Amyloid Aggregates of Alzheimer’s disease. Pharm Res 29, 3075–3089 (2012). https://doi.org/10.1007/s11095-012-0770-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0770-0