Abstract
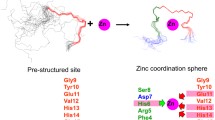
Aggregation of the amyloid β-peptide (Aβ) into insoluble fibrils is a key pathological event in Alzheimer’s disease. Cu(II) and Zn(II) ions were reported to be able to induce Aβ aggregation at nearly physiological concentrations in vitro. In this study, the binding modes of Cu(II) and Zn(II) in this process were explored by molecular modeling. In the pre-associated Aβ, Nτ atom of imidazole ring of His14, O atom of carbonyl of main-chain and two O atoms of water occupied the four ligand positions of the complex. While in the aggregated form of Aβ, the His13(N)–Metals–His14(N) bridges were formed through metal cross-linking action. These results would be helpful to put insight on revealing the formation mechanism of pathogenic Aβ aggregates in brain.




Similar content being viewed by others
References
Atwood CS, Martins RN, Smith MA, Perry G (2002) Senile plaque composition and posttranslational modification of amyloid-β peptide and associated proteins. Peptides 23:1343–1350
Atwood CS, Moir RD, Huang X et al (1998) Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem 273:12817–12826
Coles M, Bicknell W, Watson AA et al (1998) Solution structure of amyloid beta-peptide(1–40) in a water-micelle environment. Is the membrance-spanning domain where we think it is? Biochemistry 37:11064–11077
Curtain CC, Ali F, Volitakis L et al (2001) Alzheimer’s disease amyloidbeta binds copper and zinc to generate an allosterically ardered membrane penetrating structure containing superoxide dismutase-like subunits. J Biol Chem 276:20466–20473
Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR. (2003) Metal binding and oxidation of Amyloid β within isolated senile plaque cores: raman microscopic evidence. Biochemistry 42:2768–2773
Dong J, Shokes JE, Scott RA, Lynn DG (2006) Modulating amyloid self-assembly and fibril morphology with Zn(II). J Am Chem Soc 128:3540–3542
Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H (1999) Interaction between Aβ(1–42) and Aβ(1–40) in Alzheimer’s β-Amyloid fibril formation in vitro. Biochemistry 38:15514–15521
Hoops SC, Anderson KW, Merz KM (1991) Force field design for metalloproteins. J Am Chem Soc 113:8262–8270
Hou TJ, Zhang W, Xu XJ (2001) Binding affinities for a series of selective inhibitors of gelatinase––a using molecular dynamics with a linear interaction energy approach. J Phys Chem B 105:5304–5315
HyperChem Release 6.0. Hypercube Inc. 2000
Jarrett JT, Lansbury PT (1993) Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 73:1055–1058
Liu ST, Howlett G, Barrow CJ et al (1999) Histidine-13 is a crucial residue in the zinc ion-induced aggregation of the Aβ peptide of Alzheimer’s disease. Biochemistry 38:9373–9378
Lovell MA, Robertson JD, Campbell JL, Markesbery WR 1998 Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158:47–52
Luczkowski M, Wisniewska K, Kozlowski H, et al (2002) Specific binding of Cu2+ ions by a pentapeptide fragment present in the cysteine-rich region of amyloid precursor protein. J Chem Soc Dalton Trans 2266–2268
Miura T, Suzuki K, Kohata N, Takeuchi H (2000) Metal binding modes of Alzheimer’s amyloid β-peptide in insoluble aggregates and soluble complexes. Biochemistry 39:7024–7031
Morgan DM, Dong JJ, Jacob J, Lu K, Apkarian RP, Thiyagarajan P, Lynn DG (2002) Metal switch for amyloid formation: insight into the structure of the nucleus. J Am Chem Soc 124:12644–12645
Padrick SB, Miranker AD (2002) Islet amyloid: phase partitioning and secondary nucleation are central to the mechanism of fibrillogenesis. Biochemistry 41:4694–4703
Raffa DF, Gómez-Balderas R et al (2005) Ab initio model studies of copper binding to peptides containing a His–His sequence: relevance to the β-amyloid peptide of Alzheimer’s disease. J Biol Inorg Chem 10:887–902
Reichert DE, Norrby P, Welch MJ (2001) Molecular modeling of bifunctional chelate peptide conjugates. 1.Copper and indium parameters for Amber force field. Inorg Chem 40:5223–5230
Roher AE, Chaney MO, Kuo YM et al (1996) Morphology and toxicity of Aβ(1–42) dimmer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem 271:20631–20635
Stellato F, Menestrina G, Serra MD et al (2006) Metal binding in amyloid β-peptides shows intra- and inter-peptide coordination modes. Eur Biophys J 35:340–351
Storey E, Cappai R (1999) The amyloid precursor protein of Alzheimer’s disease and the Aβ peptide. Neuropathol Appl Neurobiol 25:81–97
Zou J, Kajita K, Sugimoto N (2001) Cu inhibits the aggregation of amyloid β-peptide(1–42) in vitro. Angew Chem Int Ed 40:2274–2277
Acknowledgments
The authors acknowledge the support of the Science Foundation of Xiamen University (No. Z03120), the Grand Research Foundation of Fujian Province of China (No. 2005YZ1014) and the Natural Science Foundation of Fujian Province of China (No. 2006J0184).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, D., Wang, H. & Yang, P. Molecular modeling of zinc and copper binding with Alzheimer’s amyloid β-peptide. Biometals 21, 189–196 (2008). https://doi.org/10.1007/s10534-007-9107-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-007-9107-6