ABSTRACT
Purpose
To clarify sotalol’s classification in the BCS versus BDDCS systems through cellular, rat everted sac and PAMPA permeability studies.
Methods
Studies were carried out in Madin Darby canine kidney (MDCK) and MDR1-transfected MDCK (MDCK-MDR1) cell lines, rat everted gut sacs and the Parallel Artificial Membrane Permeability Assay (PAMPA) system. Three-hour transport studies were conducted in MDCK cell lines (with apical pH changes) and MDCK-MDR1 cells (with and without the P-glycoprotein inhibitor GG918); male Sprague-Dawley rats (300~350 g) were used to prepare everted sacs. In the PAMPA studies, drug solutions at different pH’s were dosed in each well and incubated for 5 h. Samples were measured by LC-MS/MS, or liquid scintillation counting and apparent permeability (Papp) was calculated.
Results
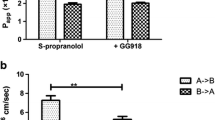
Sotalol showed low permeability in all of the cultured-cell lines, everted sacs and PAMPA systems. It might be a border line P-glycoprotein substrate. The PAMPA study showed that sotalol’s permeability increased with a higher apical pH, while much less change was found in MDCK cells.
Conclusion
The low permeability rate for sotalol correlates with its Class 3 BDDCS assignment and lack of in vivo metabolism.



Similar content being viewed by others
Abbreviations
- BCS:
-
Biopharmaceutics Classification System
- BDDCS:
-
Biopharmaceutics Drug Disposition Classification System
- FBS:
-
fetal bovine serum
- HBSS:
-
Hank’s balanced salt solution
- MDCK:
-
Madin Darby canine kidney
- MDR-1 (P-gp):
-
multidrug resistance -1 (P-glycoprotein)
- PAMPA:
-
parallel artificial membrane permeability assay
- TEER:
-
transepithelial electrical resistance
REFERENCES
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20.
Research CfDEa,U.S. Food and Drug Administration, Guidance for industry: Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system, 2000 August, http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064964.htm. (Available from)
Use CfMPfH,European Medicines Agency, Guideline on the investigation of bioequivalence, 2010 January 20, http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500070039. (Available from)
Chen M-L, Amidon GL, Benet LZ, Lennernäs H, Yu LX. The BCS, BDDCS, and regulatory guidances. Pharm Res. 2011;28:1774–8.
Wu C-Y, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23.
Benet LZ, Amidon GL, Barends D, Lennernäs H, Polli J, Shah V, et al. The use of BDDCS in classifying the permeability of marketed drugs. Pharm Res. 2008;25:483–8.
Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3:631–43.
Chen M-L, Yu LX. The use of drug metabolism for prediction of intestinal permeability. Mol Pharm. 2009;6:74–81.
Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, et al. MDCK (Madin-Darby Canine Kidney) cells: A tool for membrane permeability screening. J Pharm Sci. 1999;88:28–33.
Alt A, Potthast H, Moessinger J, Sickmüller B, Oeser H. Biopharmaceutical characterization of sotalol-containing oral immediate release drug products. Eur J Pharm Biopharm. 2004;58:145–50.
Volpe DA. Permeability classification of representative fluoroquinolones by a cell culture method. AAPS J. 2004;6:1–6.
Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. Characterization of β-adrenoceptor antagonists as substrates and inhibitors of the drug transporter p-glycoprotein. Fund Clin Pharmacol. 2006;20:273–82.
Yang Y, Faustino PJ, Volpe DA, Ellison CD, Lyon RC, Yu LX. Biopharmaceutics classification of selected β-blockers: Solubility and permeability class membership. Mol Pharm. 2007;4:608–14.
Anttila M, Arstila M, Pfeffer M, Tikkanen R, Vallinkoski V, Sundquist H. Human pharmacokinetics of sotalol. Acta Pharmacol Toxicol (Copenh). 1976;39:118–28.
Dahan A, Miller JM, Hilfinger JM, Yamashita S, Yu LX, Lennernäs H, et al. High-permeability criterion for BCS classification: Segmental/ph dependent permeability considerations. Mol Pharm. 2010;7:1827–34.
Nayak RK, Benet LZ. Drug transfer across rat intestinal musculature after edetic acid treatment. J Pharm Sci. 1971;60:1508–11.
Yamashita S, Masada M, Nadai T, Kimura T. Effect of adjuvants on charge-selective permeability and electrical resistance of rat jejunal membrane. J Pharm Sci. 1990;79:579–83.
Kato Y, Miyazaki T, Kano T, Sugiura T, Kubo Y, Tsuji A. Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009;98:2529–39.
Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–70.
Broccatelli F, Larregieu CA, Cruciani G, Oprea TI, Benet LZ. Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012; doi:10.1016/j.addr.2011.12.008.
Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, et al. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–8.
Chen X, Murawski A, Patel K, Crespi C, Balimane P. A novel design of artificial membrane for improving the PAMPA model. Pharm Res. 2008;25:1511–20.
Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia. 2006;8:143–52.
ACKNOWLEDGMENTS & DISCLOSURES
We thank the Chinese Scholarship Council for providing financial support for Wei Liu to study and carry out a portion of these studies in Dr. Benet’s laboratory at the University of California, San Francisco. The studies in Dr. Benet’s lab were funded in part by NIH grant RR031474.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, W., Okochi, H., Benet, L.Z. et al. Sotalol Permeability in Cultured-Cell, Rat Intestine, and PAMPA System. Pharm Res 29, 1768–1774 (2012). https://doi.org/10.1007/s11095-012-0699-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0699-3