Abstract
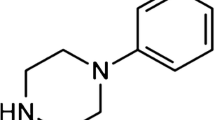
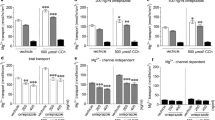
A limiting factor for oral delivery of macromolecules is low intestinal epithelial permeability. 1-Phenylpiperazine (PPZ), 1-(4-methylphenyl) piperazine (1-4-MPPZ) and 1-methyl-4-phenylpiperazine (1-M-4-PPZ) have emerged as potential permeation enhancers (PEs) from a screen carried out by others in Caco-2 monolayers. Here, their efficacy, mechanism of action and potential for epithelial toxicity were further examined in Caco-2 cells and isolated rat intestinal mucosae. Using high-content analysis, PPZ and 1-4-MPPZ decreased mitochondrial membrane potential and increased plasma membrane potential in Caco-2 cells to a greater extent than 1-M-4-PPZ. The Papp of the paracellular marker, [14C]-mannitol, and of the peptide, [3H]-octreotide, was measured across rat colonic mucosae following apical addition of the three piperazines. PPZ and 1-4-MPPZ induced a concentration-dependent decrease in transepithelial electrical resistance (TEER) and an increase in the Papp of [14C]-mannitol without causing histological damage. 1-M-4-PPZ was without effect. The piperazines caused the Krebs-Henseleit buffer pH to become alkaline, which partially attenuated the increase in Papp of [14C]-mannitol caused by PPZ and 1-4-MPPZ. Only addition of 1-4-MPPZ increased the Papp of [3H]-octreotide. Pre-incubation of mucosae with two 5-HT4 receptor antagonists, a loop diuretic and a myosin light chain kinase inhibitor, reduced the permeation enhancement capacity of PPZ and 1-4-MPP for [14C]-mannitol. 1-4-MPPZ holds most promise as a PE, but intestinal physiology may also be impacted due to multiple mechanisms of action.









Similar content being viewed by others
Abbreviations
- 1-4-MPPZ:
-
1-(4-Methylphenyl)piperazine
- 1-M-4-PPZ:
-
1-Methyl-4-phenylpiperazine
- BZP:
-
N-Benzylpiperazine
- CPE:
-
Chemical permeation enhancer
- FCCP:
-
Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- FITC:
-
Fluorescein isothiocyanate
- I sc :
-
Short circuit current
- KH:
-
Krebs-Henseleit buffer
- MDMA:
-
3,4-Methylenedioxymethamphetamine
- ML9:
-
1-(5-chloronaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine
- MLCK:
-
Myosin light chain kinase
- MMP:
-
Mitochondrial membrane potential
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium
- Na/K/2Cl:
-
Sodium/potassium/chloride cotransporter
- P app :
-
Apparent permeability coefficient
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- PMP:
-
Plasma membrane potential
- PPZ:
-
1-Phenylpiperazine
- TEER:
-
Transepithelial electrical resistance
References
Usmani SS, Bedi G, Samuel JS, Singh S, Kalra S, Kumar P, et al. THPdb: database of FDA-approved peptide and protein therapeutics. PLoS One. 2017;12:e0181748.
Maher S, Mrsny RJ, Brayden DJ. Intestinal permeation enhancers for oral peptide delivery. Adv Drug Deliv Rev. 2016;106:277–319.
Card JW, Magnuson BA. A review of the efficacy and safety of nanoparticle-based oral insulin delivery systems. Am J Physiol Liver Physiol. 2011;301:G956–67.
Abramson A, Caffarel-Salvador E, Khang M, Dellal D, Silverstein D, Gao Y, et al. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363:611–5.
Maher S, Brayden DJ, Casettari L, Illum L. Application of permeation enhancers in oral delivery of macromolecules: an update. Pharmaceutics. 2019;11:41.
Lindmark T, Nikkilä T, Artursson P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther. 1995;275:958–64.
Sonaje K, Chuang E-Y, Lin K-J, Yen T-C, Su F-Y, Tseng MT, et al. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: microscopic, ultrastructural, and computed-tomographic observations. Mol Pharm. 2012;9:1271–9.
Brayden DJ, Alonso M-J. Oral delivery of peptides: opportunities and issues for translation. Adv Drug Deliv Rev. 2016;106:193–5.
Halberg IB, Lyby K, Wassermann K, Heise T, Zijlstra E, Plum-Mörschel L. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7:179–88.
Bucheit JD, Pamulapati LG, Carter N, Malloy K, Dixon DL, Sisson EM. Oral semaglutide: a review of the first oral glucagon-like peptide-1 receptor agonist. Diabetes Technol Ther. 2019. https://doi.org/10.1089/dia.2019.0185.
Whitehead K, Karr N, Mitragotri S. Safe and effective permeation enhancers for oral drug delivery. Pharm Res. 2008;25:1782–8.
Fein KC, Lamson NG, Whitehead KA. Structure-function analysis of phenylpiperazine derivatives as intestinal permeation enhancers. Pharm Res. 2017;34:1320–9.
Martin RJ. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. Br J Pharmacol. 1982;77:255–65.
Campbell H, Cline W, Evans M, Lloyd J, Peck AW. Comparison of the effects of dexamphetamine and 1-benzylpiperazine in former addicts. Eur J Clin Pharmacol. 1973;6:170–6.
Arbo MD, Bastos ML, Carmo HF. Piperazine compounds as drugs of abuse. Drug Alcohol Depend. 2012;122:174–85.
Feuchtl A, Bagli M, Stephan R, Frahnert C, Kölsch H, Kühn K-U, et al. Pharmacokinetics of m-chlorophenylpiperazine after intravenous and oral administration in healthy male volunteers: implication for the pharmacodynamic profile. Pharmacopsychiatry. 2004;37:180–8.
Rendell M. Technosphere inhaled insulin (Afrezza). Drugs Today. 2014;50:813.
Potocka E, Cassidy JP, Haworth P, Heuman D, Van Marle S, Baughman RA. Pharmacokinetic characterization of the novel pulmonary delivery excipient fumaryl diketopiperazine. J Diabetes Sci Technol. 2010;4:1164–73.
Bzik VA, Brayden DJ. An assessment of the permeation enhancer, 1-phenyl-piperazine (PPZ), on paracellular flux across rat intestinal mucosae in Ussing chambers. Pharm Res. 2016;33:2506–16.
Dahlgren D, Sjöblom M, Lennernäs H. Intestinal absorption-modifying excipients: a current update on preclinical in vivo evaluations. Eur J Pharm Biopharm. 2019;142:411–20.
Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414.
Bunce KT, Elswood CJ, Ball MT. Investigation of the 5-hydroxytryptamine receptor mechanism mediating the short-circuit current response in rat colon. Br J Pharmacol. 1991;102:811–6.
Kellum JM, Budhoo MR, Siriwardena AK, Smith EP, Jebraili SA. Serotonin induces Cl- secretion in human jejunal mucosa in vitro via a non-neural pathway at a 5-HT4 receptor. Am J Phys. 1994;267:G357–63.
Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370–80.
Albuquerque FC, Smith EH, Kellum JM. 5-HT induces cAMP production in crypt colonocytes at a 5-HT4 receptor. J Surg Res. 1998;77:137–40.
Lamberts SWJ, Oosterom R, Neufeld M, Del Pozo E. The somatostatin analog sms 201-995 induces long-acting inhibition of growth hormone secretion without rebound hypersecretion in acromegalic patients. J Clin Endocrinol Metab. 1985;60:1161–5.
Lamberts SWJ, van der Lely A-J, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246–54.
Lancranjan I, Bruns C, Grass P, et al. Sandostatin LAR®: pharmacokinetics, pharmacodynamics, efficacy, and tolerability in acromegalic patients. Metabolism. 1995;44:18–26.
Gurel MH, Han Y, Stevens AL, Furtado A, Cox D. Treatment adherence and persistence with long-acting somatostatin analog therapy for the treatment of acromegaly: a retrospective analysis. BMC Pharmacol Toxicol. 2017;18:22.
Biermasz NR. New medical therapies on the horizon: oral octreotide. Pituitary. 2017;20:149–53.
Tuvia S, Pelled D, Marom K, Salama P, Levin-Arama M, Karmeli I, et al. A novel suspension formulation enhances intestinal absorption of macromolecules via transient and reversible transport mechanisms. Pharm Res. 2014;31:2010–21.
Brayden DJ, Gleeson J, Walsh EG. A head-to-head multi-parametric high content analysis of a series of medium chain fatty acid intestinal permeation enhancers in Caco-2 cells. Eur J Pharm Biopharm. 2014;88:830–9.
Petersen SB, Nolan G, Maher S, Rahbek UL, Guldbrandt M, Brayden DJ. Evaluation of alkylmaltosides as intestinal permeation enhancers: comparison between rat intestinal mucosal sheets and Caco-2 monolayers. Eur J Pharm Sci. 2012;47:701–12.
Shimazaki T, Tomita M, Sadahiro S, Hayashi M, Awazu S, Shimaz Aki T, et al. Absorption-enhancing effects of sodium caprate and palmitoyl carnitine in rat and human colons. Dig Dis Sci. 1998;43:641–5.
Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, et al. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol. 2018;175:987–93.
Rawlinson L-AB, O’Brien PJ, Brayden DJ. High content analysis of cytotoxic effects of pDMAEMA on human intestinal epithelial and monocyte cultures. J Control Release. 2010;146:84–92.
Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int J Cell Biol. 2010;2010:546163.
Ungell A-L, Nylander S, Bergstrand S, Sjöberg Å, Lennernäs H. Membrane transport of drugs in different regions of the intestinal tract of the rat. J Pharm Sci. 1998;87:360–6.
Lamson NG, Cusimano G, Suri K, Zhang A, Whitehead KA. The pH of piperazine derivative solutions predicts their utility as transepithelial permeation enhancers. Mol Pharm. 2016;13:578–85.
Tocris (2019) Certificate of Analysis ML9 hydrochloride. https://www.tocris.com/products/ml-9-hydrochloride_0431
O’Brien PJ. High-content analysis in toxicology: screening substances for human toxicity potential, elucidating subcellular mechanisms and in vivo use as translational safety biomarkers. Basic Clin Pharmacol Toxicol. 2014;115:4–17.
Maher S, Kennelly R, Bzik VA, Baird AW, Wang X, Winter D, et al. Evaluation of intestinal absorption enhancement and local mucosal toxicity of two promoters. I. Studies in isolated rat and human colonic mucosae. Eur J Pharm Sci. 2009;38:291–300.
Dahlgren D, Lennernäs H. Intestinal permeability and drug absorption: predictive experimental, computational and in vivo approaches. Pharmaceutics. 2019;11:411.
Nedi T, White PJ, Coupar IM, Irving HR. Tissue dependent differences in G-protein coupled receptor kinases associated with 5-HT4 receptor desensitization in the rat gastro-intestinal tract. Biochem Pharmacol. 2011;81:123–33.
Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21.
Mawe G, Hoffman J. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–86.
Hoffman JM, Tyler K, MacEachern SJ, et al. Activation of colonic mucosal 5-HT 4 receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–54.
McLean PG, Borman RA, Lee K. 5-HT in the enteric nervous system: gut function and neuropharmacology. Trends Neurosci. 2007;30:9–13.
Dotevall G, Groll E. Controlled clinical trial of mepiprazole in irritable bowel syndrome. Br Med J. 1974;4:16–8.
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–82.
Shin JJ, Saadabadi A (2019) Trazodone. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470560/
Ning Y, Jin XZ, Hsiao CC, Zhu JX, Chan HC. Regulation of ion transport by 5-hydroxytryptamine in rat colon. Clin Exp Pharmacol Physiol. 2004;31:424–8.
Hecht G, Koutsouris A. Myosin regulation of NKCC1: effects on cAMP-mediated Cl − secretion in intestinal epithelia. Am J Physiol Physiol. 1999;277:C441–7.
Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, et al. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci. 2010;107:8237–41.
Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, et al. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKCα in human intestinal epithelial cells. Gastroenterology. 2005;128:962–74.
Gandhi S, Lorimer DD, de Lanerolle P. Expression of a mutant myosin light chain that cannot be phosphorylated increases paracellular permeability. Am J Physiol Physiol. 1997;272:F214–21.
Hecht G, Pestic L, Nikcevic G, Koutsouris A, Tripuraneni J, Lorimer DD, et al. Expression of the catalytic domain of myosin light chain kinase increases paracellular permeability. Am J Physiol Physiol. 1996;271:C1678–84.
Funding
This study was funded by Science Foundation Ireland Grant 13/RC/2073, the CÚRAM Centre for Medical Devices and by the EU Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animals were used in accordance with UCD Animal Research Ethics Committee protocol for use of post-mortem tissue from animals in research (AREC 14-28-Brayden), in adherence with the “Principles of Laboratory Animal Care”, (NIH Publication #85-23, revised in 1985).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 547 kb)
Rights and permissions
About this article
Cite this article
Stuettgen, V., Brayden, D.J. Investigations of Piperazine Derivatives as Intestinal Permeation Enhancers in Isolated Rat Intestinal Tissue Mucosae. AAPS J 22, 33 (2020). https://doi.org/10.1208/s12248-020-0416-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-0416-9