ABSTRACT
Purpose
To gather sub-surface in situ images of microneedle-treated human skin, in vivo, using optical coherence tomography (OCT). This is the first study to utilise OCT to investigate the architectural changes that are induced in skin following microneedle application.
Methods
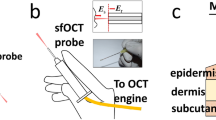
Steel, silicon and polymer microneedle devices, with different microneedle arrangements and morphologies, were applied to two anatomical sites in human volunteers following appropriate ethical approval. A state-of-the-art ultrahigh resolution OCT imaging system operating at 800 nm wavelength and <3 µm effective axial resolution was used to visualise the microneedle-treated area during insertion and/or following removal of the device, without any tissue processing.
Results
Transverse images of a microneedle device, in situ, were captured by the OCT system and suggest that the stratified skin tissue is compressed during microneedle application. Following removal of the device, the created microchannels collapse within the in vivo environment and, therefore, for all studied devices, microconduit dimensions are markedly smaller than the microneedle dimensions.
Conclusions
Microchannels created in the upper skin layers by microneedles are less invasive than previous histology predicts. OCT has the potential to play a highly influential role in the future development of microneedle devices and other transdermal delivery systems.













Similar content being viewed by others
Abbreviations
- OCT:
-
Optical coherence tomography
REFERENCES
Bos JD, Meinardi M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9.
Badkar AV, Smith AM, Eppstein JA, Banga AK. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007;24(7):1389–95.
Bramson J, Dayball K, Evelegh C, Wan YH, Page D, Smith A. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10(3):251–60.
Birchall J, Coulman S, Anstey A, Gateley C, Sweetland H, Gershonowitz A, et al. Cutaneous gene expression of plasmid DNA in excised human skin following delivery via microchannels created by radio frequency ablation. Int J Pharm. 2006;312(1–2):15–23.
Levin G, Gershonowitz A, Sacks H, Stern M, Sherman A, Rudaev S, et al. Transdermal delivery of human growth hormone through RF-microchannels. Pharm Res. 2005;22(4):550–5.
Banga AK. Microporation applications for enhancing drug delivery. Expert Opin Drug Deliv. 2009;6(4):343–54.
Gerstel MS, Place VA. Drug Delivery Device. 1976: US.
Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87(8):922–5.
McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Nal Acad Sci USA. 2003;100(24):13755–60.
Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66.
Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25(1):104–13.
Sivamani RK, Stoeber B, Wu GC, Zhai HB, Liepmann D, Maibach H. Clinical microneedle injection of methyl nicotinate: stratum corneum penetration. Skin Res Technol. 2005;11(2):152–6.
Nordquist L, Roxhed N, Griss P, Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration. Pharm Res. 2007;24(7):1381–8.
Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–52.
Li GH, Badkar A, Nema S, Kolli CS, Banga AK. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm. 2009;368(1–2):109–15.
Coulman SA, Barrow D, Anstey A, Gateley C, Morrissey A, Wilke N, et al. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr Drug Deliv. 2006;3(1):65–75.
Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97(3):503–11.
Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14(4):375–81.
Mikszta JA, Dekker JP, Harvey NG, Dean CH, Brittingham JM, Huang J, et al. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun. 2006;74(12):6806–10.
Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653–64.
Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, et al. Microneedle mediated delivery of nanoparticles into human skin. Int J Pharm. 2009;366(1–2):190–200.
Birchall J, Coulman S, Pearton M, Allender C, Brain K, Anstey A, et al. Cutaneous DNA delivery and gene expression in ex vivo human skin explants via wet-etch microfabricated microneedles. J Drug Target. 2005;13(7):415–21.
Godin B, Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 2007;59(11):1152–61.
Ng KW, Pearton M, Coulman S, Anstey A, Gateley C, Morrissey A, et al. Development of an ex vivo human skin model for intradermal vaccination: tissue viability and Langerhans cell behaviour. Vaccine. 2009;27(43):5948–55.
Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35(3):193–202.
Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009;11(1):35–47.
Sivamani RK, Stoeber B, Liepmann D, Maibach HI. Microneedle penetration and injection past the stratum corneum in humans. J Dermatol Treat. 2009;20(3):156–9.
Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27(3):454–9.
Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24(7):585–94.
Levin J, Maibach H. The correlation between transepidermal water loss and percutaneous absorption: an overview. J Control Release. 2005;103(2):291–9.
Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7(1):1–9.
Huang D, Swanson E, Lin C, Schuman J, Stinson W, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81.
Gambichler T, Boms S, Stucker M, Kreuter A, Moussa G, Sand M, et al. Epidermal thickness assessed by optical coherence tomography and routine histology: preliminary results of method comparison. J Eur Acad Dermatol. 2006;20(7):791–5.
Gambichler T, Matip R, Moussa G, Altmeyer P, Hoffmann K. In vivo data of epidermal thickness evaluated by optical coherence tomography: effects of age, gender, skin type, and anatomic site. J Dermatol Sci. 2006;44(3):145–52.
Konig K, Speicher M, Buckle R, Reckfort J, McKenzie G, Welzel J, et al. Clinical optical coherence tomography combined with multiphoton tomography of patients with skin diseases. J Biophotonics. 2009;2(6–7):389–97.
Mogensen M, Nurnberg BM, Forman JL, Thomsen JB, Thrane L, Jemec GBE. In vivo thickness measurement of basal cell carcinoma and actinic keratosis with optical coherence tomography and 20-MHz ultrasound. Br J Dermatol. 2009;160(5):1026–33.
Neerken S, Lucassen GW, Bisschop MA, Lenderink E, Nuijs TAM. Characterization of age-related effects in human skin: a comparative study that applies confocal laser scanning microscopy and optical coherence tomography. J Biomed Opt. 2004;9(2):274–81.
Querleux B, Baldeweck T, Diridollou S, de Rigal J, Huguet E, Leroy F, et al. Skin from various ethnic origins and aging: an in vivo cross-sectional multimodality imaging study. Skin Res Technol. 2009;15(3):306–13.
Welzel J, Reinhardt C, Lankenau E, Winter C, Wolff HH. Changes in function and morphology of normal human skin: evaluation using optical coherence tomography. Br J Dermatol. 2004;150(2):220–5.
Alex A, Považay B, Hofer B, Popov S, Glittenberg C, Binder S, Drexler W. Multispectral in vivo three-dimensional optical coherence tomography of human skin. J Biomed Opt. 2010;026025.
Kim CS, Wilder-Smith P, Ahn YC, Liaw LHL, Chen ZP, Kwon YJ. Enhanced detection of early-stage oral cancer in vivo by optical coherence tomography using multimodal delivery of gold nanoparticles. J Biomed Opt. 2009;14(3):034008.
Yoon J, Son T, Choi EH, Choi B, Nelson JS, Jung B. Enhancement of optical skin clearing efficacy using a microneedle roller. J Biomed Opt. 2008;13(2):021103.
Stumpp O, Welch AJ, Gill HS, Prausnitz MR. OCT analysis of microneedle and Er: YAG surface ablation for enhanced transdermal delivery of hyper-osmotic agents for optical skin clearing. Laser Interaction with Tissue and Cells Xv. 2004;5319:121–9.
Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–37.
Wilke N, Morrissey A. Silicon microneedle formation using modified mask designs based on convex corner undercut. J Micromech Microeng. 2007;17(2):238–44.
Unterhuber A, Považay B, Hermann B, Sattmann H, Drexler W, Yakovlev V, et al. Compact, low-cost Ti: Al2O3 laser for in vivo ultrahigh-resolution optical coherence tomography. Opt Lett. 2003;28(11):905–7.
Kim Y-C, Quan F-S, Yoo D-G, Compans RW, Kang S-M, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27(49):6932–8.
Laurent A, Mistretta F, Bottigioli D, Dahel K, Goujon C, Nicolas JF, et al. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine. 2007;25(34):6423–30.
Kim Y-C, Quan F-S, Compans RW, Kang S-M, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–95.
Donnelly RF, Singh TRR, Tunney MM, Morrow DIJ, McCarron PA, O’Mahony C, et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26(11):2513–22.
Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Del Rev. 2004;56(5):581–7.
Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–80.
ACKNOWLEDGEMENTS
We would like to acknowledge the support of Jessika Weingast from the Division of General Dermatology at the Department of Dermatology of the Medical University of Vienna. This research was supported in part by the BBSRC, Cardiff University, FP6-IST-NMP-2 STREPT (017128, NanoUB), DTI grant (OMICRON), AMR grant (AP1110), European Union project FUN OCT (FP7 HEALTH, contract no. 201880) and CARL ZEISS Meditec Inc.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Video 1
(AVI 9077 kb)
ESM Video 2
(AVI 9976 kb)
Rights and permissions
About this article
Cite this article
Coulman, S.A., Birchall, J.C., Alex, A. et al. In Vivo, In Situ Imaging of Microneedle Insertion into the Skin of Human Volunteers Using Optical Coherence Tomography. Pharm Res 28, 66–81 (2011). https://doi.org/10.1007/s11095-010-0167-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0167-x