Abstract
Purpose
To develop a rational basis for designing coating solution formulations for uniform and thick coatings on microneedles and to identify coating strategies to form composite coatings, deliver liquid formulations, and control the mass deposited on microneedles.
Materials and Methods
Microneedles were fabricated using laser-cutting and then dip-coated using different aqueous, organic solvent-based or molten liquid formulations. The mass of riboflavin (vitamin B2) coated onto microneedles was determined as a function of coating and microneedle parameters. Coated microneedles were also inserted into porcine cadaver skin to assess delivery efficacy.
Results
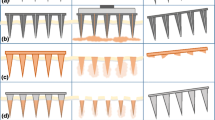
Sharp-tipped microneedles, including pocketed microneedles, were fabricated. Excipients that reduced coating solution surface tension improved coating uniformity, while excipients that increased solution viscosity improved coating thickness. Evaluation of more than 20 different coating formulations using FDA approved excipients showed that hydrophilic and hydrophobic molecules could be uniformly coated onto microneedles. Model proteins were also uniformly coated on microneedles using the formulations identified in the study. Pocketed microneedles were selectively filled with solid or liquid formulations to deliver difficult-to-coat substances, and composite drug layers were formed for different release profiles. The mass of riboflavin coated onto microneedles increased with its concentration in the coating solution and the number of coating dips and microneedles in the array. Coatings rapidly dissolved in the skin without wiping off on the skin surface.
Conclusions
Microneedles and coating formulations can be designed to have a range of different properties to address different drug delivery scenarios.







Similar content being viewed by others
References
M. R. Prausnitz, S. Mitragotri, and R. Langer. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 3:115–124 (2004).
M. R. Prausnitz. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 56:581–587 (2004).
M. Prausnitz, J. Mikszta, and J. Raeder-Devens. Microneedles. In E. W. Smith and H. I. Maibach (eds.), Percutaneous Penetration Enhancers, CRC, Boca Raton, FL, 2005, pp. 239–255.
M. L. Reed and W.-K. Lyev. Microsystems for drug and gene delivery. Proc. IEEE 92:56–75 (2004).
S. Henry, D. V. McAllister, M. G. Allen, and M. R. Prausnitz. Microfabricated microneedles: a novel approach to transdermal drug delivery. J. Pharm. Sci. 87:922–925 (1998).
D. V. McAllister, P. M. Wang, S. P. Davis, J. H. Park, P. J. Canatella, M. G. Allen, and M. R. Prausnitz. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc. Natl. Acad. Sci. U. S. A. 100:13755–13760 (2003).
F. Chabri, K. Bouris, T. Jones, D. Barrow, A. Hann, C. Allender, K. Brain, and J. Birchall. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br. J. Dermatol. 150:869–877 (2004).
H. J. G. E. Gardeniers, R. Luttge, E. J. W. Berenschot, M. J. De Boer, S. Y. Yeshurun, M. Hefetz, R. Van’t Oever, and A. Van Den Berg. Silicon micromachined hollow microneedles for transdermal liquid transport. J. MEMS. 12:855–862 (2003).
R. K. Sivamani, B. Stoeber, G. C. Wu, H. Zhai, D. Liepmann, and H. Maibach. Clinical microneedle injection of methyl nicotinate: stratum corneum penetration. Skin Res. Technol. 11:152–156 (2005).
W. Martanto, S. P. Davis, N. R. Holiday, J. Wang, H. S. Gill, and M. R. Prausnitz. Transdermal delivery of insulin using microneedles in vivo. Pharm. Res. 21:947–952 (2004).
M. Cormier, B. Johnson, M. Ameri, K. Nyam, L. Libiran, D. D. Zhang, and P. Daddona. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 97:503–511 (2004).
J. A. Mikszta, J. B. Alarcon, J. M. Brittingham, D. E. Sutter, R. J. Pettis, and N. G. Harvey. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 8:415–419 (2002).
W. Lin, M. Cormier, A. Samiee, A. Griffin, B. Johnson, C. L. Teng, G. E. Hardee, and P. E. Daddona. Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux) technology. Pharm. Res. 18:1789–1793 (2001).
J. A. Mikszta, V. J. Sullivan, C. Dean, A. M. Waterston, J. B. Alarcon, J. P. Dekker, J. M. III, Brittingham, J. Huang, C. R. Hwang, M. Ferriter, G. Jiang, K. Mar, K. U. Saikh, B. G. Stiles, C. J. Roy, R. G. Ulrich, and N. G. Harvey. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J. Infect. Dis. 191:278–288 (2005).
H. S. Gill and M. R. Prausnitz. Coated microneedles for transdermal delivery. J. Control. Release 117:227–237 (2007).
J. A. Matriano, M. Cormier, J. Johnson, W. A. Young, M. Buttery, K. Nyam, and P. E. Daddona. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm. Res. 19:63–70 (2002).
G. Widera, J. Johnson, L. Kim, L. Libiran, K. Nyam, P. E. Daddona, and M. Cormier. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine 24:1653–1664 (2006).
S. Yoshioka and V. J. Stella. Stability of Drugs and Dosage Forms, Kluwer, New York, 2002.
R. J. Stokes, D. F. Evans, and M. Errico. Liquid coating processes. In R. J. Stokes and D. F. Evans (eds.), Fundamentals of Interfacial Engineering, Wiley-VCH, Weinheim, Germany, 1997, pp. 399–456.
T. D. Blake and K. J. Ruschak. Wetting: static and dynamic contact lines. In S. F. Kistler and P. M. Schweizer (eds.), Liquid Film Coating : Scientific Principles and Their Technological Implications, Chapman & Hall, London, 1997, pp. 63–97.
L. E. Scriven. Physics and applications of dip coating and spin coating. In C. J. Brinker, D. E. Clark, and D. R. Ulrich (eds.), Mat Res Soc Symp Proc, Vol. 121, Materials Research Society, 1988, pp. 717–729.
H. S. Kheshgi. The fate of thin liquid films after coating. In S. F. Kistler and P. M. Schweizer (eds.), Liquid Film Coating : Scientific Principles and Their Technological Implications, Chapman & Hall, London, 1997, pp. 183–672.
D. R. Lide. CRC Handbook of Chemistry and Physics, 87th edition, CRC, Boca Raton, FL, 2006.
Q. Zhao, C. Wang, Y. Liu, and S. Wang. Bacterial adhesion on the metal-polymer composite coatings. Int. J. Adhes. Adhes. 27:85–91 (2007).
A. H. Kibbe (ed.). Handbook of Pharmaceutical Excipients, American Pharmaceutical Association, Washington, D.C., 2000.
S. Cheboyina, J. O’Haver, and C.M. Wyandt. A mathematical model to predict the size of the pellets formed in freeze pelletization techniques: parameters affecting pellet size. J. Pharm. Sci. 95:167–180 (2006).
C. Leuner and J. Dressman. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 50:47–60 (2000).
Draft Guidance for Industry on Powder Blends and Finished Dosage Units–Stratified In-Process Dosage Unit Sampling and Assessment, Docket no. 2003D-0493, Federal Drug Adminstration, Rockville, MD, 2003.
Acknowledgment
We would like to thank Dr. Mark Allen for use of the IR and CO2 lasers in his lab; Richard Shafer, Dr. Shawn Davis, and Ed Birdsell for helpful discussions regarding laser operation; and Dr. Jung-Hwan Park for making silicon dioxide coatings on microneedles. MRP is the Emerson-Lewis Faculty Fellow. This work was supported in part by the National Institutes of Health and took place in the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gill, H.S., Prausnitz, M.R. Coating Formulations for Microneedles. Pharm Res 24, 1369–1380 (2007). https://doi.org/10.1007/s11095-007-9286-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9286-4