Abstract
Purpose
To compare the ability of alkyl-aryl isothiocyanates (ITCs) to increase the activities of the Phase 2 detoxification enzymes NAD[P]H:quinone acceptor oxidoreductase 1 (NQO1) and glutathione S-transferases (GST) in rat tissues in vivo and in cells in vitro.
Materials and Methods
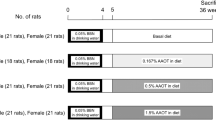
Twelve alkyl-aryl ITCs and the fully-reduced derivative of benzyl ITC (cyclohexylmethyl ITC) were administered to rats each day for 5 days. The animals were then killed and organs harvested. The ITCs were also evaluated in a bladder cell line in culture. The activities of NQO1 and GST in the organs and cells were measured.
Results
In vivo, the organ most susceptible to the inductive activity of the ITCs was the urinary bladder, with α-methylbenzyl ITC and cyclohexylmethyl ITC being the most effective. Inductive activity in the bladder in vivo did not, however, correlate with that in bladder cells in vitro.
Conclusions
Induction of Phase 2 enzymes increases resistance to chemical carcinogenesis. ITCs could therefore be valuable chemopreventative agents, and the specificity of these substances toward the urinary bladder suggest that they could be particularly useful for protecting against bladder cancer. In this regard, α-methylbenzyl ITC and cyclohexylmethyl ITC could be especially valuable.




Similar content being viewed by others
References
J. T. Wilkinson, M. A. Morse, L. A. Kresty, and G. D. Stoner. Effect of alkyl chain length on inhibition of N-nitrosomethylbenzylamine-induced esophagal tumorigenesis and DNA methylation by isothiocyanates. Carcinogenesis. 16:1011–1015 (1995).
G. D. Stoner, C. Adams, L. A. Kresty, S. G. Amin, D. Desai, S. S. Hecht, S. E. Murphy, and M. A. Morse. Inhibition of N’-nitrosonornicotine-induced esophageal tumorigenesis by 3-phenylpropyl isothiocyanate. Carcinogenesis. 1998:2139–2143 (1998).
M. A. Morse, K. I. Eklind, S. G. Amin, S. S. Hecht, and F.-L. Chung. Effect of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis. 10:1757–1759 (1989).
A. Nishikawa, F. Furukawa, C. Uneyama, S. Ikezaki, Z.-Y. Tanakamaru, and Y. Hayashi. Chemopreventative effects of phenethyl isothiocyanate on lung and pancreatic tumorigenesis in N-nitrosobis(2-oxopropyl)amine-treated hamsters. Carcinogenesis. 17:1381–1384 (1996).
S. Sugie, A. Okumura, T. Tanaka, and H. Mori. Inhibitory effects of benzyl isothiocyanate and benzyl thiocyanate on diethylnitrosamine-induced hepatocarcinogenesis in rats. Jpn. J. Cancer Res. 84:865–870 (1993).
S. Sugie, K. Okamoto, A. Okumura, T. Tanaka, and H. Mori. Inhibitory effects of benzyl thiocyanate and benzyl isothiocyanate on methylazoxymethanol acetate-induced intestinal carcinogenesis. Carcinogenesis. 15:1555–1560 (1994).
F.-L. Chung, C. C. Conaway, C. V. Rao, and B. S. Reddy. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 21:2287–2291 (2000).
L. W. Wattenberg. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J. Nat. Cancer Inst. 58:395–398 (1977).
Y. Zhang. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat. Res. 555:173–190 (2004).
R. Munday, Y. Zhang, J. W. Fahey, H. E. Jobson, C. M. Munday, J. Li, and K. K. Stephenson. Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: Critical nature of in vivo bioassay. Nutr. Cancer. 54:223–231 (2006).
J. D. Hayes, and D. J. Pulford. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 30:445–600 (1995).
A. T. Dinkova-Kostova, and P. Talalay. Persuasive evidence that quinone reductase Type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Rad. Biol. Med. 29:231–240 (2000).
J. Šalagovič, I. Kalina, V. Habalová, M. Hrivňák, L. Valanský, and E. Biroš. The role of human glutathione S-transferases M1 and T1 in individual susceptibility to bladder cancer. Physiol. Res. 48:465–471 (1999).
W. A. Schulz, A. Krummeck, I. Rösinger, P. Eickelmann, C. Neuhaus, T. Ebert, B. J. Schmitz-Dräger, and H. Sies. Increased frequency of a null-allele for NAD(P)H:quinone oxidoreductase in patients with urological malignancies. Pharmacogenetics. 7:235–239 (1997).
S.-J. Park, H. Zhao, M. R. Spitz, H. B. Grossman, and X. Wu. An association between NQO1 polymorphism and risk of bladder cancer. Mutat. Res. 536:131–137 (2003).
G. A. Törüner, C. Akyerli, A. Uçar, T. Aki, N. Atsu, H. Ozen, M. Tez, M. Cetinkaya, and T. Ozçelik. Polymorphisms of glutathione S-transferase genes (GSTM1, GSTP1 and GSTT1) and bladder cancer susceptibility in the Turkish population. Arch. Toxicol. 75:459–464 (2001).
R. Munday, P. Mhawech-Fauceglia, C. M. Munday, J. D. Paonessa, L. Tang, J. S. Munday, C. Lister, P. Wilson, J. W. Fahey, W. Davis, and Y. Zhang. Inhibition of urinary bladder carcinogenesis by broccoli sprout extracts. Cancer Res. 68:1593–1600 (2008).
J. W. Fahey, A. T. Zalcmann, and P. Talalay. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 56:5–51 (2001).
A. M. Benson, P. B. Barreto, and J. S. Stanley. Induction of DT-diaphorase by anticarcinogenic sulfur compounds in mice. J. Nat. Cancer Inst. 76:467–473 (1986).
G.-Q. Zheng, P. M. Kenney, and L. K. T. Lam. Phenylalkyl isothiocyanate-cysteine conjugates as glutathione S-transferase stimulating agents. J. Med. Chem. 35:185–188 (1992).
L. Drobnica, P. Kristián, and J. Augustin. The chemistry of the-NCS group. In S. Patai (ed.), The Chemistry of Cyanates and their Thio Derivatives, Part 2, Wiley, Chichester, UK, 1977, pp. 1003–1221.
L. Ernster. DT diaphorase. Meth. Enzymol. 10:309–317 (1967).
W. H. Habig, M. J. Pabst, and W. B. Jakoby. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249:7130–7139 (1974).
G. Brüsewitz, B. D. Cameron, L. F. Chasseaud, K. Görler, D. R. Hawkins, H. Koch, and W. H. Mennicke. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem. J. 162:99–107 (1977).
C. C. Conaway, J. Krzeminski, S. Amin, and F. L. Chung. Decomposition rates of isothiocyanate conjugates determine their activity as inhibitors of cytochrome p450 enzymes. Chem. Res. Toxicol. 14:1170–1176 (2001).
L. Tang, and Y. Zhang. Isothiocyanates in the chemoprevention of bladder cancer. Curr. Drug Metab. 5:193–201 (2004).
R. Munday, and C. M. Munday. Selective induction of Phase II enzymes in the urinary bladder of rats by allyl isothiocyanate, a compound derived from Brassica vegetables. Nutr. Cancer. 44:52–59 (2002).
Y. Zhang, R. Munday, H. E. Jobson, C. M. Munday, C. Lister, P. Wilson, J. W. Fahey, and P. Mhawech-Fauceglia. Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts. J. Agric. Food Chem. 54:9370–9376 (2006).
Y. Zhang, and P. Talalay. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Res. 58:4632–4639 (1998).
R. Munday, and C. M. Munday. Induction of Phase II detoxification enzymes in rats by plant-derived isothiocyanates: Comparison of allyl isothiocyanate with sulforaphane and related compounds. J. Agric. Food Chem. 52:1867–1871 (2004).
A. Nishikawa, M. A. Morse, and F.-L. Chung. Inhibitory effects of 2-mercaptoethane sulfonate and 6-phenylhexyl isothiocyanate on urinary bladder tumorigenesis in rats induced by N-butyl-N-(4-hydroxybutyl)nitrosamine. Cancer Lett. 193:11–16 (2003).
K. Okazaki, M. Yamagishi, H.-Y. Son, T. Imazawa, F. Furukawa, H. Nakamura, A. Nishikawa, T. Masegi, and M. Hirose. Simultaneous treatment with benzyl isothiocyanate, a strong bladder promoter, inhibits rat urinary bladder carcinogenesis by N-butyl-N-(4-hydroxybutyl)nitrosamine. Nutr. Cancer. 42:211–216 (2002).
K. Okazaki, T. Umemura, T. Imazawa, A. Nishikawa, T. Masegi, and M. Hirose. Enhancement of urinary bladder carcinogenesis by combined treatment with benzyl isothiocyanate and N-butyl-N-(4-hydroxybutyl)nitrosamine in rats after initiation. Cancer Sci. 94:948–952 (2003).
K. Ogawa, M. Futakuchi, M. Hirose, P. Boonyaphiphat, Y. Mizoguchi, T. Miki, and T. Shirai. Stage and organ dependent effects of 1-O-hexyl-2,3,5-trimethylhydroquinone, ascorbic acid derivatives, N-heptadecane-8,10-dione and phenylethyl isothiocyanate in a rat multiorgan carcinogenesis model. Int. J. Cancer. 76:851–856 (1998).
M. Hirose, T. Yamaguchi, N. Kimoto, K. Ogawa, M. Futakuchi, M. Sano, and T. Shirai. Strong promoting activity of phenylethyl isothiocyanate and benzyl isothiocyanate on urinary bladder carcinogenesis in F344 male rats. Int. J. Cancer. 77:773–777 (1998).
S. Sugiura, K. Ogawa, M. Hirose, F. Takashita, M. Asamoto, and T. Shirai. Reversibility of proliferative lesions and induction of non-papillary tumors in rat urinary bladder treated with phenylethyl isothiocyanate. Carcinogenesis. 24:547–553 (2003).
H. Takagi, M. Shibutani, C. Uneyama, K.-Y. Lee, N. Kato, K. Inoue, and M. Hirose. Limited tumor-initiating activity of phenylethyl isothiocyanate by promotion with sodium L-ascorbate in a rat two-stage urinary bladder carcinogenesis model. Cancer Lett. 219:147–153 (2005).
NCI, DCPC Chemoprevention Branch and Agent Development Committee. Clinical development plan: phenethyl isothiocyanate. J. Cell Biochem. 26S:149–157 (1996).
R. A. Lubet, V. E. Steele, I. Eto, M. M. Juliana, G. J. Kelloff, and C. J. Grubbs. Chemopreventive efficacy of anethole trithione, N-acetyl-L-cysteine, miconazole and phenethylisothiocyanate in the DMBA-induced rat mammary cancer model. Int. J. Cancer. 72:95–101 (1997).
Acknowledgements
This work was funded by the Waikato Medical Research Foundation (New Zealand) and National Cancer Institute Grant CA 80962 (USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munday, R., Zhang, Y., Munday, C.M. et al. Structure–Activity Relationships and Organ Specificity in the Induction of GST and NQO1 by Alkyl-Aryl Isothiocyanates. Pharm Res 25, 2164–2170 (2008). https://doi.org/10.1007/s11095-008-9595-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9595-2