Abstract
Purpose
Melittin is an amphipathic antimicrobial peptide which has been shown to enhance the permeability of mannitol and reduce transepithelial electrical resistance (TER) across Caco-2 monolayers. The aim of this work was to further examine the potential of melittin as a paracellular permeability enhancer and to investigate the mechanism of interaction with tight junction proteins in Caco-2.
Materials and Methods
The permeability of a range of fluorescent markers of differing molecular weights across monolayers was examined and immunofluorescence and western blotting analysis of tight junction proteins were also carried out. The mechanism of TER reduction was also examined using cell signalling inhibitors.
Results
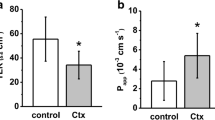
Apical but not basolateral addition of melittin increased the permeability of a range FITC-dextrans (4–70 kDa) across monolayers. Melittin effects were reversible and no cytotoxicity was evident in polarized Caco-2 epithelia at the concentrations used. Altered expression of ZO-1, E-cadherin and F-actin was also detected. The phospholipase A2 inhibitors, aristolochic acid and indomethacin and the cyclooxygenase inhibitor, piroxicam, partially attenuated melittin-induced TER reduction, suggesting that part of the mechanism by which melittin opens tight junctions involves prostaglandin signalling.
Conclusions
Apically-added melittin opens tight junctions, causing dramatic TER reductions with significant increases in flux of dextrans. These effects appear mediated in part via PLA2 and involve alterations in specific tight junction proteins.







Similar content being viewed by others
References
R. J. Mrsny. Modification of epithelial tight junction integrity to enhance transmucosal absorption. Crit. Rev. Ther. Drug Carr. Syst. 22:331–418 (2005).
M. J. Cano-Cebrian, T. Zornoza, L. Granero, and A. Polache. Intestinal absorption enhancement via the paracellular route by fatty acids, chitosans and others: a target for drug delivery. Curr. Drug. Deliv. 2:9–22 (2005).
N. A. Motlekar, K. S. Srivenugopal, M. S. Wachtel, and B. B. Youan. Oral delivery of low-molecular-weight heparin using sodium caprate as absorption enhancer reaches therapeutic levels. J. Drug Target. 13:573–583 (2005).
P. D. Ward, T. K. Tippin, and D. R. Thakker. Enhancing paracellular permeability by modulating epithelial tight junctions. Pharm. Sci. Technol. Today 3:346–358 (2000).
T. Lindmark, J. D. Soderholm, G. Olaison, G. Alvan, G. Ocklind, and P. Artursson. Mechanism of absorption enhancement in humans after rectal administration of ampicillin in suppositories containing sodium caprate. Pharm. Res. 14:930–935 (1997).
T. W. Leonard, J. Lynch, M. J. McKenna, and D. J. Brayden. Promoting absorption of drugs in humans using medium-chain fatty acid-based solid dosage forms: GIPET. Expert Opin. Drug Deliv. 3:685–692 (2006).
N. S. Harhaj and D. A. Antonetti. Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 36:1206–1237 (2004).
D. A. Mann and A. D. Frankel. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 10:1733–1739 (1991).
M. S. Dilber, A. Phelan, A. Aints, A. J. Mohamed, G. Elliott, C. I. Smith, and P. O’Hare. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein, VP22. Gene Ther. 6:12–21 (1999).
A. Fasano and S. Uzzau. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J. Clin. Invest. 99:1158–1164 (1997).
I. E. Andras, H. Pu, M. A. Deli, A. Nath, B. Hennig, and M. Toborek. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J. Neurosci. Res. 74:255–265 (2003).
D. Allende, S. A. Simon, and T. J. McIntosh. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophys. J. 88:1828–1837 (2005).
L. Yang, T. A. Harroun, T. M. Weiss, L. Ding, and H. W. Huang. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 81:1475–1485 (2001).
P. Liu, P. Davis, H. Liu, and T. R. Krishnan. Evaluation of cytotoxicity and absorption enhancing effects of melittin—a novel absorption enhancer. Eur. J. Pharm. Biopharm. 48:85–87 (1999).
S. Maher and S. McClean. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem. Pharmacol. 71:1289–1298 (2006).
M. T. Tosteson, S. J. Holmes, M. Razin, and D. C. Tosteson. Melittin lysis of red cells. J. Membr. Biol. 87:35–44 (1985).
P. M. Hwang and H. J. Vogel. Structure-function relationships of antimicrobial peptides. Biochem. Cell. Biol. 76:235–246 (1998).
W. S. Foster and H. W. Jarrett. Melittin-silica, a high-pressure affinity chromatography resin for calmodulin. J. Chromatogr. 403:99–107 (1987).
K. J. Baker, J. M. East, and A. G. Lee. Mechanism of inhibition of the Ca(2+)-ATPase by melittin. Biochemistry 34:3596–3604 (1995).
J. Cuppoletti and D. H. Malinowska. Interaction of polypeptides with the gastric (H+ + K+)ATPase: melittin, synthetic analogs, and a potential intracellular regulatory protein. Mol. Cell. Biochem. 114:57–63 (1992).
K. R. Gravitt, N. E. Ward, and C. A. O’Brian. Inhibition of protein kinase C by melittin: antagonism of binding interactions between melittin and the catalytic domain by active-site binding of MgATP. Biochem. Pharmacol. 47:425–427 (1994).
H. K. Paudel, Y. H. Xu, H. W. Jarrett, and G. M. Carlson. The model calmodulin-binding peptide melittin inhibits phosphorylase kinase by interacting with its catalytic center. Biochemistry 32:11865–11872 (1993).
B. H. Knowles and R. W. Farndale. Activation of insect cell adenylate cyclase by Bacillus thuringiensis delta-endotoxins and melittin. Toxicity is independent of cyclic AMP. Biochem. J. 253:235–241 (1988).
M. Diener and W. Rummel. Phospholipase A2 and mediation of the activation of short-circuit current in the rat colonic mucosa. Naunyn-Schmiedeberg’s Arch. Pharmacol. 343:652–658 (1991).
P. Artursson and J. Karlsson. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 175:880–885 (1991).
T. Lindmark, N. Schipper, L. Lazorova, A. G. de Boer, and P. Artursson. Absorption enhancement in intestinal epithelial Caco-2 monolayers by sodium caprate: assessment of molecular weight dependence and demonstration of transport routes. J. Drug Target. 5:215–223 (1998).
T. Suzuki and H. Hara. Difructose anhydride III and sodium caprate activate paracellular transport via different intracellular events in Caco-2 cells. Life Sci. 79:401–410 (2006).
C. J. Watson, M. Rowland, and G. Warhurst. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am. J. Physiol., Cell Physiol. 281:C388–C397 (2001).
M. Tomita, M. Hayashi, and S. Awazu. Absorption-enhancing mechanism of EDTA, caprate, and decanoylcarnitine in Caco-2 cells. J. Pharm. Sci. 85:608–611 (1996).
S. Maher, L. Feighery, D. J. Brayden, and S. McClean. Melittin as a permeability enhancer II: in vitro investigations in human mucus secreting intestinal monolayers and rat colonic mucosae. Pharm. Res. (2007, in press). DOI 10.1007/s11095-007-9246-z
D. Brayden, E. Creed, A. O’Connell, H. Leipold, R. Agarwal, and A. Leone-Bay. Heparin absorption across the intestine: effects of sodium N-[8-(2-hydroxybenzoyl)amino]caprylate in rat in situ intestinal instillations and in Caco-2 monolayers. Pharm. Res. 14:1772–1779 (1997).
L. Garrity-Ryan, B. Kazmierczak, R. Kowal, J. Comolli, A. Hauser, and J. N. Engel. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68:7100–7113 (2000).
J. M. Anderson, C. M. Van Itallie, M. D. Peterson, B. R. Stevenson, E. A. Carew, and M. S. Mooseker. ZO-1 mRNA and protein expression during tight junction assembly in Caco-2 cells. J. Cell Biol. 109:1047–1056 (1989).
N. G. Schipper, S. Olsson, J. A. Hoogstraate, A. G. deBoer, K. M. Varum, and P. Artursson. Chitosans as absorption enhancers for poorly absorbable drugs 2: mechanism of absorption enhancement. Pharm. Res. 14:923–929 (1997).
L. Illum. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 15:1326–1331 (1998).
T. Lindmark, Y. Kimura, and P. Artursson. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J. Pharmacol. Exp. Ther. 284:362–369 (1998).
A. Fasano. Modulation of intestinal permeability: an innovative method of oral drug delivery for the treatment of inherited and acquired human diseases. Mol. Genet. Metab. 64:12–18 (1998).
G. Hecht, L. Pestic, G. Nikcevic, A. Koutsouris, J. Tripuraneni, D. D. Lorimer, G. Nowak, V. Guerriero, Jr., E. L. Elson, and P. D. Lanerolle. Expression of the catalytic domain of myosin light chain kinase increases paracellular permeability. Am. J. Physiol. 271:C1678–C1684 (1996).
A. Fasano, C. Fiorentini, G. Donelli, S. Uzzau, J. B. Kaper, K. Margaretten, X. Ding, S. Guandalini, L. Comstock, and S. E. Goldblum. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J. Clin. Invest. 96:710–720 (1995).
K. Koumanov, A. Momchilova, and C. Wolf. Bimodal regulatory effect of melittin and phospholipase A2-activating protein on human type II secretory phospholipase A2. Cell Biol. Int. 27:871–877 (2003).
T. Sawai, N. Usui, J. Dwaihy, R. A. Drongowski, A. Abe, A. G. Coran, and C. M. Harmon. The effect of phospholipase A2 on bacterial translocation in a cell culture model. Pediatr. Surg. Int. 16:262–266 (2000).
R. Martin-Venegas, S. Roig-Perez, R. Ferrer, and J. J. Moreno. Arachidonic acid cascade and epithelial barrier function during Caco-2 cell differentiation. J. Lipid Res. 47:1416–1423 (2006).
A. Ohata, M. Usami, and M. Miyoshi. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 21:838–847 (2005).
C. Celik-Ozenci, I. Ustunel, T. Erdogru, Y. Seval, E. T. Korgun, M. Baykara, and R. Demir. Ultrastructural and immunohistochemical analysis of rat uroepithelial cell junctions after partial bladder outlet obstruction and selective COX-2 inhibitor treatment. Acta Histochem. 107:443–451 (2006).
W. G. Jiang, R. P. Bryce, D. F. Horrobin, and R. E. Mansel. Regulation of tight junction permeability and occludin expression by polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 244:414–420 (1998).
J. R. Turner, J. M. Angle, E. D. Black, J. L. Joyal, D. B. Sacks, and J. L. Madara. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am. J. Physiol. 277:C554–C562 (1999).
J. R. Turner, B. K. Rill, S. L. Carlson, D. Carnes, R. Kerner, R. J. Mrsny, and J. L. Madara. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 273:C1378–C1385 (1997).
L. Pang, M. Nie, L. Corbett, R. Donnelly, S. Gray, and A. J. Knox. Protein kinase C-epsilon mediates bradykinin-induced cyclooxygenase-2 expression in human airway smooth muscle cells. FASEB J. 16:1435–1437 (2002).
Acknowledgements
This work was supported by the Programme for Research in Third Level Institutions (PRTLI) administered by HEA, Ireland. LF was funded by the Health Research Board (Ireland). The authors express their gratitude to James Reilly, ITT Dublin for advice on statistical analysis of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maher, S., Feighery, L., Brayden, D.J. et al. Melittin as an Epithelial Permeability Enhancer I: Investigation of Its Mechanism of Action in Caco-2 Monolayers. Pharm Res 24, 1336–1345 (2007). https://doi.org/10.1007/s11095-007-9288-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9288-2