Purpose
The mechanism of intestinal drug transport for hydrophilic cations such as ranitidine is complex, and evidence suggests a role for carrier-mediated apical (AP) uptake and saturable paracellular mechanisms in their overall absorptive transport. The purpose of this study was to develop a model capable of describing the kinetics of cellular accumulation and transport of ranitidine in Caco-2 cells, and to assess the relative contribution of the transcellular and paracellular routes toward overall ranitidine transport.
Methods
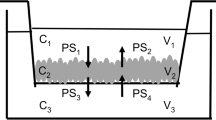
Cellular accumulation and absorptive transport of ranitidine were determined in the absence or presence of uptake and efflux inhibitors and as a function of concentration over 60 min in Caco-2 cells. A three-compartment model was developed, and parameter estimates were utilized to assess the expected relative contribution from transcellular and paracellular transport.
Results
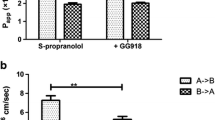
Under all conditions, ranitidine absorptive transport consisted of significant transcellular and paracellular components. Inhibition of P-glycoprotein decreased the AP efflux rate constant (k 21) and increased the relative contribution of the transcellular transport pathway. In the presence of quinidine, both the AP uptake rate constant (k 12) and k 21 decreased, resulting in a predominantly paracellular contribution to ranitidine transport. Increasing the ranitidine donor concentration decreased k 12 and the paracellular rate constant (k 13). No significant changes were observed in the relative contribution of the paracellular and transcellular routes as a function of ranitidine concentration.
Conclusions
These results suggest the importance of uptake and efflux transporters as determinants of the relative contribution of transcellular and paracellular transport for ranitidine, and provide evidence supporting a concentration-dependent paracellular transport mechanism. The modeling approach developed here may also be useful in estimating the relative contribution of paracellular and transcellular transport for a wide array of drugs expected to utilize both pathways.






Similar content being viewed by others
Abbreviations
- AP:
-
apical
- BL:
-
basolateral
- k 12 :
-
AP uptake rate constant
- k 13 :
-
paracellular rate constant
- k 21 :
-
AP efflux rate constant
- k 23 :
-
BL efflux rate constant
- k 32 :
-
BL uptake rate constant
- P app,para :
-
paracellular permeability
- P app,total :
-
total permeability
- P app,trans :
-
transcellular permeability
- P-gp:
-
P-glycoprotein
References
D. L. Bourdet and D. R. Thakker. Saturable absorptive transport of the hydrophilic organic cation ranitidine in caco-2 cells: role of pH-dependent organic cation uptake system and P-glycoprotein. Pharm. Res. 23(6): (2006).
L. S. Gan P. H. Hsyu J. F. Pritchard D. Thakker (1993) ArticleTitleMechanism of intestinal absorption of ranitidine and ondansetron: transport across Caco-2 cell monolayers Pharm. Res. 10 1722–1725 Occurrence Handle8302757 Occurrence Handle1:CAS:528:DyaK2cXmvVyhsg%3D%3D Occurrence Handle10.1023/A:1018965929419
A. Collett N. B. Higgs E. Sims M. Rowland G. Warhurst (1999) ArticleTitleModulation of the permeability of H2 receptor antagonists cimetidine and ranitidine by P-glycoprotein in rat intestine and the human colonic cell line Caco-2 J. Pharmacol. Exp. Ther. 288 171–178 Occurrence Handle9862768 Occurrence Handle1:CAS:528:DyaK1MXisVyrsw%3D%3D
K. Lee D. R. Thakker (1999) ArticleTitleSaturable transport of H2-antagonists ranitidine and famotidine across Caco-2 cell monolayers J. Pharm. Sci. 88 680–687 Occurrence Handle10393565 Occurrence Handle1:CAS:528:DyaK1MXjt1Sjtr0%3D Occurrence Handle10.1021/js980474k
A. Adson T. J. Raub P. S. Burton C. L. Barsuhn A. R. Hilgers K. L. Audus N. F. Ho (1994) ArticleTitleQuantitative approaches to delineate paracellular diffusion in cultured epithelial cell monolayers J. Pharm. Sci. 83 1529–1536 Occurrence Handle7891269 Occurrence Handle1:CAS:528:DyaK2cXmtlyht7k%3D
A. Adson P. S. Burton T. J. Raub C. L. Barsuhn K. L. Audus N. F. Ho (1995) ArticleTitlePassive diffusion of weak organic electrolytes across Caco-2 cell monolayers: uncoupling the contributions of hydrodynamic, transcellular, and paracellular barriers J. Pharm. Sci. 84 1197–1204 Occurrence Handle8801334 Occurrence Handle1:CAS:528:DyaK2MXnvFSrs7s%3D
V. Pade S. Stavchansky (1997) ArticleTitleEstimation of the relative contribution of the transcellular and paracellular pathway to the transport of passively absorbed drugs in the Caco-2 cell culture model Pharm. Res. 14 1210–1215 Occurrence Handle9327450 Occurrence Handle1:CAS:528:DyaK2sXmsFehurY%3D Occurrence Handle10.1023/A:1012111008617
R. Saitoh K. Sugano N. Takata T. Tachibana A. Higashida Y. Nabuchi Y. Aso (2004) ArticleTitleCorrection of permeability with pore radius of tight junctions in Caco-2 monolayers improves the prediction of the dose fraction of hydrophilic drugs absorbed by humans Pharm. Res. 21 749–755 Occurrence Handle15180329 Occurrence Handle1:CAS:528:DC%2BD2cXjs1Kisrk%3D Occurrence Handle10.1023/B:PHAM.0000026423.48583.e2
N. Nagahara S. Tavelin P. Artursson (2004) ArticleTitleContribution of the paracellular route to the pH-dependent epithelial permeability to cationic drugs J. Pharm. Sci. 93 2972–2984 Occurrence Handle15459946 Occurrence Handle1:CAS:528:DC%2BD2cXhtVKlsrvN Occurrence Handle10.1002/jps.20206
P. Schmiedlin-Ren K. E. Thummel J. M. Fisher M. F. Paine K. S. Lown P. B. Watkins (1997) ArticleTitleExpression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1alpha,25-dihydroxyvitamin D3 Mol. Pharmacol. 51 741–754 Occurrence Handle9145912 Occurrence Handle1:CAS:528:DyaK2sXjt1ehtr0%3D
F. Hyafil C. Vergely P. Vignaud ParticleDu T. Grand-Perret (1993) ArticleTitleIn vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative Cancer. Res. 53 4595–4602 Occurrence Handle8402633 Occurrence Handle1:CAS:528:DyaK2cXitlWnsA%3D%3D
K. Lee C. Ng K. L. R. Brouwer D. R. Thakker (2002) ArticleTitleSecretory transport of ranitidine and famotidine across Caco-2 cell monolayers J. Pharmacol. Exp. Ther. 303 574–580 Occurrence Handle12388638 Occurrence Handle1:CAS:528:DC%2BD38XotlCgu70%3D Occurrence Handle10.1124/jpet.102.038521
M. F. Fromm R. B. Kim C. M. Stein G. R. Wilkinson D. M. Roden (1999) ArticleTitleInhibition of P-glycoprotein-mediated drug transport: a unifying mechanism to explain the interaction between digoxin and quinidine Circulation 99 552–557 Occurrence Handle9927403 Occurrence Handle1:CAS:528:DyaK1MXht1Gnsr4%3D
G. T. Knipp N. F. Ho C. L. Barsuhn R. T. Borchardt (1997) ArticleTitleParacellular diffusion in Caco-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size J. Pharm. Sci. 86 1105–1110 Occurrence Handle9344165 Occurrence Handle1:CAS:528:DyaK2sXlvVygsb8%3D Occurrence Handle10.1021/js9700309
O. R. Colegio C. M. Itallie ParticleVan H. J. McCrea C. Rahner J. M. Anderson (2002) ArticleTitleClaudins create charge-selective channels in the paracellular pathway between epithelial cells Am. J. Physiol. Cell Physiol. 283 C142–C147 Occurrence Handle12055082 Occurrence Handle1:CAS:528:DC%2BD38XlsVCrsrw%3D
N. Piyapolrungroj Y. S. Zhou C. Li G. Liu E. Zimmermann D. Fleisher (2000) ArticleTitleCimetidine absorption and elimination in rat small intestine Drug Metab. Dispos. 28 65–72 Occurrence Handle10611142 Occurrence Handle1:CAS:528:DC%2BD3cXis1ymsQ%3D%3D
S. Y. Zhou N. Piyapolrungroj L. Pao C. Li G. Liu E. Zimmermann D. Fleisher (1999) ArticleTitleRegulation of paracellular absorption of cimetidine and 5-aminosalicylate in rat intestine Pharm. Res. 16 1781–1785 Occurrence Handle10571287 Occurrence Handle1:CAS:528:DyaK1MXnt1GgsLc%3D Occurrence Handle10.1023/A:1018974519984
L. S. Gan S. Yanni D. R. Thakker (1998) ArticleTitleModulation of the tight junctions of the Caco-2 cell monolayers by H2-antagonists Pharm. Res. 15 53–57 Occurrence Handle9487546 Occurrence Handle1:CAS:528:DyaK1cXosV2qtw%3D%3D Occurrence Handle10.1023/A:1011944602662
P. Artursson A. L. Ungell J. E. Lofroth (1993) ArticleTitleSelective paracellular permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments Pharm. Res. 10 1123–1129 Occurrence Handle8415396 Occurrence Handle1:CAS:528:DyaK3sXmtFKks74%3D Occurrence Handle10.1023/A:1018903931777
C. M. Brandquist C. Ng D. R. Thakker K. L. Brouwer (2003) ArticleTitleNovel interactions in the oral absorption of hydrophilic cations: a clinical study with ranitidine and famotidine Clin. Pharmacol. Ther. 73 19 Occurrence Handle10.1016/S0009-9236(03)90425-5
Acknowledgments
David L. Bourdet was supported by a Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation predoctoral fellowship in pharmaceutics. The Caco-2 cell line was kindly provided by Drs. Mary F. Paine and Paul Watkins of the University of North Carolina at Chapel Hill.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bourdet, D.L., Pollack, G.M. & Thakker, D.R. Intestinal Absorptive Transport of the Hydrophilic Cation Ranitidine: A Kinetic Modeling Approach to Elucidate the Role of Uptake and Efflux Transporters and Paracellular vs. Transcellular Transport in Caco-2 Cells. Pharm Res 23, 1178–1187 (2006). https://doi.org/10.1007/s11095-006-0204-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-0204-y