Abstract
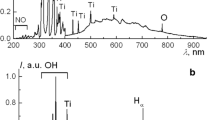
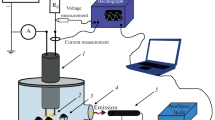
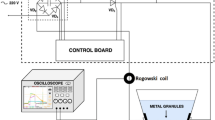
Solution plasma is a promising method for the production of nanostructured materials. The explanation of physical and electrochemical processes during the growth of nanostructures under the action of solution plasma is very important for understanding the mechanism of oxide nanostructures formation. When forming nanoparticles of noble metals, some processes on electrodes are not considered due to the properties of these materials. However, near-electrode processes can play the main role in the synthesis of the oxide structures from non-noble metals.






Similar content being viewed by others
References
Saito N, Hieda J, Takai O (2009) Synthesis process of gold nanoparticles in solution plasma. Thin Solid Films 518:912–917
Höfft O, Endres F (2011) Plasma electrochemistry in ionic liquids: an alternative route to generate nanoparticles. Phys Chem Chem Phys 13:13472–13478
Kareem TA, Kaliani AA (2012) Glow discharge plasma electrolysis for nanoparticles synthesis. Ionics 18:315–327
Bratescu MA, Takai O, Saito N (2013) One-step synthesis of gold bimetallic nanoparticles with various metal-compositions. J Alloys Compds 562:74–83
Shirai N, Uchida S, Tochikubo F (2014) Synthesis of metal nanoparticles by dual plasma electrolysis using atmospheric dc glow discharge in contact with liquid. Japan J Appl Phys 53:046202
Pootawang P, Saito N, Takai O, Lee SY (2012) Synthesis and characteristics of Ag/Pt bimetallic nanocomposites by arc-discharge solution plasma processing. Nanotechnol 23:395602
Shutov DA, Smirnova KV, Gromov MV, Ivanov AN, Rybkin VV (2018) Synthesis of CdO ultradisperse powders using atmospheric pressure glow discharge in contact with solution and the investigation of intermediate products. Plasma Chem Plasma Process 38:107–121
Sirotkin NA, Khlyustova AV, Titov VA, Krayev AS, Nikitin DI, Dmitrieva OA, Agafonov AV (2020) Synthesis and photocatalytic activity of WO3 nanoparticles prepared by underwater impulse discharge. Plasma Chem Plasma Process 40:571–587
Ashkarran AA, Kavianipour M, Aghigh SM, Afshar SA, Saviz S, Zad AI (2010) On the formation of TiO2 nanoparticles via submerged arc discharge technique: synthesis, characterization and photocatalytic properties. J Cluster Sci 21:753–766
Feng G, Wu B, Khan AQ, Zeng H (2018) In situ glow discharge plasma electrolytic synthesis of reduced TiO2 for enhanced visible light photocatalysis. Mater Res Exp 5:055022
Khlyustova AV, Sirotkin NA, Titov VA, Agafonov AV (2020) Comparison of two types of plasma in contact with water during the formation of molybdenum oxide. Curr Appl Phys 20:1396–1403
Khlyustova AV, Sirotkin NA, Kraev AS, Titov VA, Agafonov AV (2021) Synthesis and characterization of titanium oxide nanoparticles by plasma in contact with liquid. Plasma Chem Plasma Process 41:643–657
Sirotkin NA, Gurina DL, Khlyustova AV, Costerin DY, Naumova IK, Titov VA, Agafonov AV (2021) Experimental and computational investigation of polylactic acid/silver-NP nanocomposite with antimicrobial activity prepared by plasma in liquid. Plasma Process Polym 18:2000169
Khan AQ, Yuan S, Niu S, Zheng L, Li W, Zeng H (2017) Synthesis of molybdenum oxide-titanium dioxide nanocomposites with ultrashort laser ablation in water. Opt Exp 25:A539–A546
Zhang D, Gokce B, Barcikowski S (2017) Laser synthesis and processing of colloids: fundamentals and applications. Chem Rev 117:3990–4103
Burakov VS, Nevar EA, Nedel’ko MI, Tarasenko NV (2015) Synthesis and modification of molecular nanoparticles in electrical discharge plasma in liquids. Russ J Gen Chem 85:1222–1237
Fehlner FP (1986) Low temperature oxidation, the role of vitreous oxides. NY, Wiley,
Takahashi H, Umehara Y, Miyamoto T, Fujimoto N, Nagayama M (1987) Anodizing of aluminium covered with hydroxide I. Formation of hydroxide and composite oxide. films J Met Finish Soc Jpn 38:67
Bakovets VV, Polyakov OV, Dolgovesova IP (1991) Plasma-electrolytic anode treatment of metals,Novosibirsk, Science, (in rus).
Bratescu MA, Cho SP, Takai O, Saito N (2011) Size-controlled gold nanoparticles synthesized in solution plasma. J Phys Chem C 115:24569–24576
Saito G, Hosokai S, Tsubota M, Akiyama T (2011) Synthesis of copper/copper oxide nanoparticles by solution plasma. J Appl Phys 110:023302
Saito G, Sakaguchi N (2015) Solution plasma synthesis of Si nanoparticles. Nanotechnol 26:235602
Taqieddin A, Nazari R, Rajic L, Alshawabkeh A (2017) Physicochemical hydrodynamics of gas bubbles in two phase electrochemical systems. J Electrochem Soc 164:E448
Bunkin NF, Suyazov NV, Shkirin AV, Ignat’ev PS, Indukaev KV (2009) Cluster structure of stable dissolved gas nanobubbles in highly purified water. J Exp Theor Phys 108:800–816
Khlyustova AV, Sirotkin NA, Kraev AS, Titov VA, Agafonov AV (2021) Parameters of underwater plasma as a factor determining the structure of oxides (Al, Cu, and Fe). Materialia 16:101081
Farajimotlagh M, Poursalehi R, Aliofkhazraei M (2017) Synthesis mechanisms, optical and structural properties of η-Al2O3 based nanoparticles prepared by DC arc discharge in environmentally friendly liquids. Ceram Int 43:7717–7723
Khlyustova AV, Sirotkin NA, Titov VA, Agafonov AV (2021) Effect of low-temperature underwater plasma produced of new properties of Mo–Ti mixed oxide composites for electron transport layer in the dye-sensitized solar cells. J Alloys Compnds 858:157664
Hur TB, Phuoc TX, Chyu MK (2010) New approach to the synthesis of layered double hydroxides and associated ultrathin nanosheets in de-ionized water by laser ablation. J Appl Phys 108:114312
Khlyustova A, Sirotkin N, Titov V, Agafonov A (2022) One-pot underwater plasma synthesis and characterization of Fe‐and Ni‐doped boehmite. Cryst Res Technol 57:2100117
Amrute AP, Łodziana Z, Schreyer H, Weidenthaler C, Schüth F (2019) High-surface-area corundum by mechanochemically induced phase transformation of boehmite. Science 366:485–489
Acknowledgements
Authors acknowledge the Dr. N. Fomina for conducting the SEM analysis, Dr. Yu. Belikova for conducting the TEM analysis and prof. A. Agafonov for fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sirotkin, N., Khlyustova, A. The Oxide Nanostructures Formation Mechanisms in Underwater Plasma in Terms of Electrochemistry. Plasma Chem Plasma Process 42, 1003–1013 (2022). https://doi.org/10.1007/s11090-022-10263-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-022-10263-1