Abstract
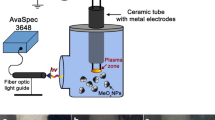
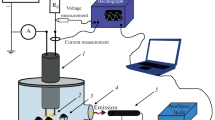
Two types of plasma in contact with water were employed for the synthesis of mixed-phase TiO2 in a liquid. This is glow discharge with a metal cathode and water anode and the underwater plasma. The plasmas were characterized by the optical emission spectroscopy. To detect the reactive species in the liquid phase the chemical dosimetry was used. X-ray diffraction, BET analysis, and scanning electron microscopy were used to characterize obtained powders. The powders obtained by both types of plasma were an anatase-rutile mixture. The rutile content was higher in the sample, which synthesized by an underwater plasma. The sample obtained by the glow discharge had a more developed surface. The data on the sorption ability of the obtained powders for metal ions and organic dyes are presented. The advantages and limitations of using plasma in contact with water for the synthesis of titanium dioxide are considered.




Similar content being viewed by others
References
Malekshahi Byranvand M, Nemati Kharat A, Fatholahi L, Malekshahi Beiranvand Z (2013) A review on synthesis of nano-TiO2 via different methods. J Nanostruct 3:1–9
Scanlon DO, Dunnill CW, Buckeridge J, Shevlin SA, Logsdail AJ, Woodley SM, Catlow CRA, Powell MJ, Palgrave RG, Parkin IP, Watson GW, Kael TW, Sherwood P, Walsh A, Sokol AA (2013) Band alignment of rutile and anatase TiO2. Nat Mater 12:798–801
Li S, Chen J, Zheng F, Li Y, Huang F (2013) Synthesis of the double-shell anatase–rutile TiO2 hollow spheres with enhanced photocatalytic activity. Nanoscale 5:12150–12155
Lei J, Li H, Zhang J, Anpo M (2016) Mixed-phase TiO2 nanomaterials as efficient photocatalysts. In: Low-dimensional and nanostructured materials and devices. Springer, Cham, pp 423–460
Xu H, Li G, Zhu G, Zhu K, Jin S (2015) Enhanced photocatalytic degradation of rutile/anatase TiO2 heterojunction nanoflowers. Catal Commun 62:52–56
Lee KY, Sato K, Mohamed AR (2016) Facile synthesis of anatase-rutile TiO2 composites with enhanced CO2 photoreduction activity and the effect of Pt loading on product selectivity. Mater Lett 163:240–243
Shigeta M, Murphy AB (2011) Thermal plasmas for nanofabrication. J Phys D Appl Phys 44:174025
Seo JH, Hong BG (2012) Thermal plasma synthesis of nano-sized powders. Nucl Eng Technol 44:9–20
Kim TH, Jeong SJ, Lim HR, Cho HB, Lee CG, Choa YH (2018) Bulk-direct synthesis of TiO2 nanoparticles by plasma-assisted electrolysis with enhanced photocatalytic performance. J Electrochem Soc 165:E64–E69
Li JG, Kamiyama H, Wang XH, Moriyoshi Y, Ishigaki T (2006) TiO2 nanopowders via radio-frequency thermal plasma oxidation of organic liquid precursors: synthesis and characterization. J Eur Ceram Soc 26:423–428
Hattori Y, Nomura S, Mukasa S, Toyota H, Inoue T, Usui T (2013) Synthesis of tungsten oxide, silver, and gold nanoparticles by radio frequency plasma in water. J Alloys Compd 578:148–152
Dhamale GD, Mathe VL, Bhoraskar SV, Sahasrabudhe SN, Dhole SD, Ghorui S (2016) Synthesis and characterization of Nd2O3 nanoparticles in a radiofrequency thermal plasma reactor. Nanotechnology 27:085603
Xu Y, Zhang Y, He T, Ding K, Huang X, Li H, Shi J, Guo Y, Zhang J (2019) The effects of thermal and atmospheric pressure radio frequency plasma annealing in the crystallization of TiO2 thin films. Coatings 9:357
Oh SM, Ishigaki T (2004) Preparation of pure rutile and anatase TiO2 nanopowders using RF thermal plasma. Thin Solid Films 457:186–191
Ashkarran AA, Kavianipour M, Aghigh SM, Afshar SA, Saviz S, Zad AI (2010) On the formation of TiO2 nanoparticles via submerged arc discharge technique: synthesis, characterization and photocatalytic properties. J Clust Sci 21:753–766
Nakasugi Y, Saito G, Yamashita T, Akiyama T (2014) Synthesis of nonstoichiometric titanium oxide nanoparticles using discharge in HCl solution. J Appl Phys 115:123303
Jedsukontorn T, Ueno T, Saito N, Hunsom M (2017) Facile preparation of defective black TiO2 through the solution plasma process: effect of parametric changes for plasma discharge on its structural and optical properties. J Alloys Compd 726:67–577
Zhang X, Zhang L, Li Y, Di L (2015) Atmospheric-pressure cold plasma for fabrication of anatase–rutile mixed TiO2 with the assistance of ionic liquid. Catal Today 256:215–220
Chu SZ, Inoue S, Wada K, Hishita S, Kurashima K (2005) Self-organized nanoporous anodic titania films and ordered titania nanodots/nanorods on glass. Adv Funct Mater 15:1343–1349
Sreekantan S, Wei LC, Lockman Z (2011) Extremely fast growth rate of TiO2 nanotube arrays in electrochemical bath containing H2O2. J Electrochem Soc 158:C397–C402
Feng G, Wu B, Khan AQ, Zeng H (2018) In situ glow discharge plasma electrolytic synthesis of reduced TiO2 for enhanced visible light photocatalysis. Mater Res Exp 5:055022
Sirotkin NA, Khlyustova AV, Titov VA, Krayev AS, Nikitin DI, Dmitrieva OA, Agafonov AV (2020) Synthesis and photocatalytic activity of WO3 nanoparticles prepared by underwater impulse discharge. Plasma Chem Plasma Proc 40:571–587
Khlyustova A, Sirotkin N, Kraev A, Titov V, Agafonov A (2020) Plasma–liquid synthesis of MoOx and WO3 as potential photocatalysts. Dalton Trans 49:6270
Khlyustova AV, Titov VA (2015) Formation rate and energy yield of hydrated electrons at the gas discharge treatment of water. Prikladnaja Fizika 6:48 (in Russian)
Khlyustova A, Khomyakova N, Sirotkin N, Marfin Y (2016) The effect of pH on OH radical generation in aqueous solutions by atmospheric pressure glow discharge. Plasma Chem Plasma Proc 36:1229–1238
Khlyustova A, Sirotkin N, Evdokimova O, Prysiazhnyi V, Titov V (2018) Efficacy of underwater AC diaphragm discharge in generation of reactive species in aqueous solutions. J Electrost 96:76–84
Eisenberg G (1943) Colorimetric determination of hydrogen peroxide. Ind Eng Chem Anal Ed 15:327–328
Griess P (1879) Bemerkungen zu der Abhandlung der HH. Ber dtsch chem 12:426–428
Harris DC (2010) Quantitative chemical analysis.7th edn. W.H. Freeman and Company, New York
Shen VK, Siderius DW, Krekelberg WP, Hatch HW (eds) NIST standard reference simulation website, NIST standard reference database number 173. National Institute of Standards and Technology, Gaithersburg, MD, p 20899. https://doi.org/10.18434/T4M88Q
Saito G, Nakasugi Y, Akiyama T (2014) Excitation temperature of a solution plasma during nanoparticle synthesis. J Appl Phys 116:083301
Hontañón E, Palomares JM, Stein M, Guo X, Engeln R, Nirschl H, Kruis FE (2013) The transition from spark to arc discharge and its implications with respect to nanoparticle production. J Nanopart Res 15:1957
Miron C, Bratescu MA, Saito N, Takai O (2010) Time-resolved optical emission spectroscopy in water electrical discharges. Plasma Chem Plasma Proc 30:619–631
Sahni M, Locke BR (2006) The effect of reaction conditions on liquid-phase hydroxyl-radical production in gas-liquid pulsed-electrical-discharge reactors. Plasma Process Polym 3:668–681
IUPAC SC-database. www.acadsoft.co.uk
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reaction of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/Oˉ) in aqueous solution. J Phys Chem Ref Data 17:513–886
Rumbach P, Bartels DM, Sankaran RM, Go DB (2015) The solvation of electrons by an atmospheric-pressure plasma. Nat Commun 6:1–7
Rumbach P, Bartels DM, Sankaran RM, Go DB (2015) The effect of air on solvated electron chemistry at a plasma/liquid interface. J Phys D Appl Phys 48:424001
De Baerdemaeker F, Šimek M, Člupek M, Lukeš P, Leys C (2006) Hydrogen peroxide production in capillary underwater discharges. Czechoslov J Phys 56:B1132
De Baerdemaeker F, Šimek M, Leys C (2007) Efficiency of hydrogen peroxide production by ac capillary discharge in water solution. J Phys D Appl Phys 40:2801
Hong YC, Huh JY, Ma SH, Kim KI (2018) Inactivation of microorganisms by radical droplets from combination of water discharge and electro-spraying. J Electrost 91:56–60
Hsein KC, Wandell RJ, Bresh S, Locke BR (2017) Analysis of hydroxyl radical formation in a gas liquid electrical discharge plasma reactor utilizing liquid and gaseous radical scavengers. Plasma Process Polym 14:1600171
Crystallography open database. www.cryctallography.net. Accessed 04 May 2020
Xu L, Garrett MP, Hu B (2012) Doping effects on internally coupled seebeck coefficient, electrical, and thermal conductivities in aluminum-doped TiO2. J Phys Chem C 116:13020–13025
Hanaor DAH, Sorrell CC (2011) Review of the anatase to rutile phase transformation. J Mater Sci 46:855–874
Zhang H, Banfield JF (2000) Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2. J Phys Chem B 104:3481–3487
Galizia P, Maizza G, Galassi C (2016) Heating rate dependence of anatase to rutile transformation. Process Appl Ceram 10:235–241
Khlyustova AV, Sirotkin NA, Krayev AS, Titov VA, Agafonov AV (2019) Synthesis of MoO3 by glow discharge in contact with water. Plasma Sci Technol 21:025505
Laegreid N, Wehner GK (1961) Sputtering yields of metals for Ar+ and Ne+ ions with energies from 50 to 600 eV. J Appl Phys 32:365–369
Gerasimova TV, Evdokimova OL, Kraev AS, Ivanov VK, Agafonov AV (2016) Micro-mesoporous anatase TiO2 nanorods with high specific surface area possessing enhanced adsorption ability and photocatalytic activity. Microporous Mesoporous Mater 235:185–194
Liang P, Qin Y, Hu B, Li C, Peng T, Jiang Z (2000) Study of the adsorption behavior of heavy metal ions on nanometer-size titanium dioxide with ICP-AES. Fresenius J Anal Chem 368:638–640
Prasertsung I, Kaewcharoen S, Kunpinit K, Yaowarat W, Saito N, Phenrat T (2019) Enhanced degradation of methylene blue by a solution plasma process catalyzed by incidentally co-generated copper nanoparticles. Water Sci Technol 79:967–974
Acknowledgements
This work was performed in the frame of the Government Assignment of the Ministry of Education and Science of Russia (no. 0092-2019-0004) and was in part supported by the Russian Science Foundation under grant 19-73-00022. Authors would like to thank the Dr. N. Fomina for conducting XRD analysis and Dr. N. Kochkina for conducting DLS measurements at the center of joint use of scientific equipment (the Upper Volga Regional Center for Physical-Chemical Research, Russia) and the Dr. A. Ovtsyn for conducting SEM analysis (Interdepartmental Laboratory of Structural Analysis Methods at the Ivanovo State University of Chemistry and Technology).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Khlyustova, A.V., Sirotkin, N.A., Kraev, A.S. et al. Synthesis and Characterization of Titanium Oxide Nanoparticles by Plasma in Contact with Liquid. Plasma Chem Plasma Process 41, 643–657 (2021). https://doi.org/10.1007/s11090-020-10136-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10136-5