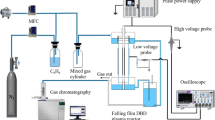
Abstract
The effect of the structure of VOC and the exposed surface area on the decomposition of VOCs adsorbed on the zeolite by dielectric barrier discharge plasma were investigated. The decomposition mechanisms of heptane and toluene, which are chosen as a model reaction for alkane and aromatic VOC, respectively, were compared each other at the same experimental conditions in the plasma reactor having the segmented electrode. The comparison was discussed in terms of mineralization, CO2 selectivity, ozone consumption, by-products, and energy yield. Also, the exposed surface area was expected to have a close relationship with the ozone consumption. The segmented electrode reactor could accelerate the dehydrogenation reaction at the low temperature as well as the atmospheric pressure without any support of catalyst. In each decomposition mechanism, the formation of hydrocarbon radical was considered as a key stage for decomposition of VOCs. The hydrocarbon radicals in the heptane decomposition were formed by the dehydrogenation, but that in the toluene decomposition were formed by the ring cleavage. The formation of hydrocarbon radicals could increase the active spots which could be attacked by the ozone, leading to the increased use efficiency of ozone. In conclusion, the toluene was decomposed easily when the similar energy was applied because the number of hydrogen atom was fewer.











Similar content being viewed by others
References
Harrison PT (2002) Indoor air quality guidelines. Occup Environ Med 59:73–74
Al-Hemoud A, Al-Awadi L, Al-Khayat A, Behbehani W (2018) Streamlining IAQ guidelines and investigation the effect of door opening/closing on concentrations of VOCs, formaldehyde, and NO2 in office buildings. Build Environ 137:127–137
Lewnadowski DA (1999) Design of thermal oxidation systems for volatile organic compounds. Lewis Publishers, New York
Warahena ASK, Chuah YK (2009) Energy recovery efficiency and cost analysis of VOC thermal oxidation pollution control technology. Environ Sci Technol 43:6101–6105
Mustafa MF, Fu X, Liu Y, Abbas Y, Wang H (2018) Volatile organic compounds (VOCs) removal in non-thermal plasma double dielectric barrier discharge reactor. J Hazard Mater 347:317–324
Karatoum O, Deshusses MA (2016) A comparative study of dilute VOCs treatment in a non-thermal plasma reactor. Chem Eng J 294:308–315
Ragazzi M, Tosi P, Rada EC, Torretta V, Schiavon M (2014) Effluents from MBT plants: plasma techniques for the treatments of VOCs. Waste Manag (Oxford) 34:2400–2406
Jiang L, Li S, Cheng Z, Chen J, Nie G (2018) Treatment of 1,2-dichloroethane and n-hexane in a combined system of non-thermal plasma catalysis reactor coupled with a biotricking filter. J Chem Technol Biotechnol 93:127–137
Azlah N, Shareefdeen Z, Elkamel A (2017) Dispersion of volatile organic compounds (VOCs) emissions from a biofilter at an electronic manufacturing facility. Environ Prog Sustain Energy 36:1100–1107
Ribeiro BMB, Pinto JF, Suppinno RS, Marcola L, Landers R, Tomaz E (2019) Catalytic oxidation at pilot-scale: efficient degradation of volatile organic compounds in gas phase. J Hazard Mater 365:581–589
Saoud WA, Assadi AA, Guiza M, Bouaza A, Aboussaoud W, Quedemi A, Soultrel I, Wolbert D, Rtimi S (2017) Study of synergetic effect, catalytic poisoning and regeneration using dielectric barrier discharge and photocatalysis in a continuous reactor: abatement of pollutants in air mixture system. Appl Catal B 213:53–61
Dizbay-Onat M, Floyd E, Vaidya UK, Lungu CT (2018) Applicability of industrial sisal fiber waste derived activated carbon for the adsorption of volatile organic compounds (VOCs). Fiber Polym 19:805–811
Hu MM, Emamipour H, Jojnsen DL, Rood MJ, Song L, Zhang Z (2017) Monitoring and control of an adsorption system using electrical properties of the adsorbent for organic compound abatement. Environ Sci Technol 51:7581–7589
Dou B, Wang Ch, Jia Q, Li J (2013) Discharge characteristics and abatement of volatile organic compounds using plasma reactor packed with ceramic Raschig rings. J Electrostat 71:939–944
Tang X, Feng F, Ye L, Zhang X, Huang Y, Liu Z, Yan K (2013) Removal of dilute VOCs in air by post-plasma catalysis over Ag-based composite oxide catalysts. Catal Today 211:39–43
Cheng Y, Hea H, Yang C, Zeng G, Li X, Chen H, Yu G (2016) Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol Adv 34:1091–1102
Zhu T, Li RR, Ma MF, Li X (2017) Influence of energy efficiency on VOCs decomposition in non-thermal plasma reactor. Int J Environ Sci Technol 14:1505–1512
Guo T, Du X, Peng Z, Xu L, Dong J, Li J, Cheng P, Zhou Z (2017) Quantification and risk assessment of organic products resulting from non-thermal plasma removal of toluene in nitrogen. Rapid Commun Mass Spectrom 31:1424–1430
Costa G, Assadi AA, Ghaida SGA, Bouzaza A, Wolbert D (2017) Study of butyraldehyde degradation and by-products formation by using a surface plasma discharge in pilot scale: process modeling and simulation of relative humidity effect. Chem Eng J 307:785–792
Shahna FG, Bahrami A, Alimohamamadi I, Yarahmadi R, Jaleh B, Gandomi M, Ebrahimi H, Abedi KAD (2017) Chlorobenzene degeradation by non-thermal plasma combined with EG–TiO2/ZnO as a photocatalyst: effect of photocatalyst on CO2 selectivity and byproducts reduction. J Hazard Mater 324:544–553
Xu X, Wu J, He M, Fu M, Chen L, Zhu A, Ye D (2017) High-efficiency non-thermal plasma-catalysis of cobalt incorporated mesoporous MCM-41 for toluene removal. Catal Today 281:527–533
Gómez-Ramírez A, Montoro-Damas AM, Rodríguez MA, González-Elipe AR, Cotrino J (2017) Improving the pollutant removal efficiency of packed-bed plasma reactors incorporating ferroelectric components. Chem Eng J 314:311–319
Lee BJ, Kim DW, Park DW (2019) Dielectric barrier discharge reactor with the segmented electrodes for decomposition of toluene adsorbed on bare-zeolite. Chem Eng J 357:188–197
Yao S, Weng S, Jin Q, Lu H, Wu Z, Zhang X, Han J, Lu H, Tang X, Jiang B (2016) Mechanism of decane decomposition in a pulsed dielectric barrier discharge reactor. IEEE Trans Plasma Sci 44:2660–2666
Starikovskiy A, Aleksandrov N (2013) Plasma-assisted ignition and combustion. Prog Energy Combust Sci 39:61–110
Corma A (1997) From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev 97:2373–2420
Martens JA, Tielen M, Jacobs PA, Weitkamp J (1984) Estimation of the void structure and pore dimensions of molecular sieve zeolites using the hydroconversion of n-decane. Zeolites 4:98–107
Schmidt M, Jõgi I, Hołub M, Brandenburg R (2015) Non-thermal plasma based decomposition of volatile organic compounds in industrial exhaust gases. Int J Environ Sci Technol 12:3745–3754
Assadi AA, Bouzaza A, Wolbert D (2016) Comparative study between laboratory and large pilot scales for VOC’s removal from gas streams in continuous flow surface discharge plasma. Chem Eng Res Des 106:308–314
Wang X, Guo M, Zhang R, Ningr P, Ma Y, Ma Q, Wang L (2018) Removal of low-concentration thiophene by DC corona discharge plasma. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-018-3669-4
Saleem F, Zhang K, Harvey A (2019) Plasma-assisted decomposition of a biomass gasification tar analogue into lower hydrocarbons in a synthetic product gas using a dielectric barrier discharge reactor. Fuel 235:1412–1419
Zhu F, Li X, Zhang H, Wu A, Yan J, Ni M, Zhang H, Buekens A (2016) Destruction of toluene by rotating gliding arc discharge. Fuel 176:78–85
Oh SM, Kim HH, Ogata A, Einaga H, Futamura S, Pakr DW (2005) Effect of zeolite in surface discharge plasma on the decomposition of toluene. Catal Lett 99:101–104
Huang R, Lu M, Wang P, Chen Y, Wu J, Fu M, Chen L, Ye D (2015) Enhancement of the non-thermal plasma-catalytic system with different zeolites for toluene removal. RSC Adv 5:72113–72120
Kim HH, Ogata A, Futamura S (2007) Complete oxidation of volatile organic compounds (VOCs) using plasma-driven catalysis and oxygen plasma. Int J Plasma Environ Sci Technol 1:46–51
Acknowledgements
This work was supported by the Regional Innovation Center for Environmental Technology of Thermal Plasma (RIC-ETTP) at Inha University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, B., Kim, DW. & Park, DW. Decomposition of Heptane by Dielectric Barrier Discharge (DBD) Plasma Reactor Having the Segmented Electrode: Comparison of Decomposition Mechanisms to Toluene. Plasma Chem Plasma Process 40, 61–77 (2020). https://doi.org/10.1007/s11090-019-10024-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-10024-7