Abstract
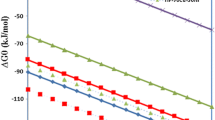
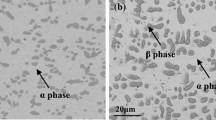
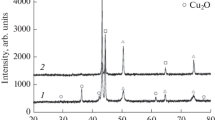
Experimental results of a new method of production of CuCo alloy of composition 95Cu–5Co from the reduction of the mixture of cupric oxide (CuO) and cobalt oxide (Co3O4) is presented. It uses low-temperature hydrogen plasma which in turn is created in a microwave assisted plasma set-up. The microwave power and hydrogen flow-rate used in this investigation are 750 W and 2.5 × 10−6 m3 s−1 respectively. Co3O4 reduced faster than CuO. The reduced molar volume of Co3O4 provided more space for hydrogen penetration. The addition of Co3O4 to CuO, not only removed the induction period from the kinetic plot of CuO reduction but also, improved the reduction rate of CuO. The kinetic data fits the Avrami model of nucleation and growth with a model parameter closer to 1.5. The alloy showed positive deviation from Vegard’s law. The crystallite size, calculated by applying Scherrer’s formula lies in the range of 21.5–30.7 nm.






Similar content being viewed by others
References
Xiao K, Qi X, Bao Z et al (2013) CuFe, CuCo and CuNi nanoparticles as catalysts for higher alcohol synthesis from syngas: a comparative study. Catal Sci Technol 3:1591–1602. https://doi.org/10.1039/c3cy00063j
Bonetti E, Del Bianco L, Savini L et al (1999) Structural configuration and magnetic properties of the rapidly solidified CuCo alloy. Nanostruct Mater 12:891–894. https://doi.org/10.1016/s0965-9773(99)00260-3
Aizawa T, Zhou C (2000) Nanogranulation process into magneto-resistant Co–Cu alloy on the route of bulk mechanical alloying. Mater Sci Eng A 285:1–7. https://doi.org/10.1016/s0921-5093(00)00709-7
Gómez E, Labarta A, Llorente A, Vallés E (2002) Characterisation of cobalt/copper multilayers obtained by electrodeposition. Surf Coat Technol 153:261–266. https://doi.org/10.1016/s0257-8972(01)01698-x
Tochitskii TA, Jones GA, Blythe HJ et al (2001) Fine structure and possible growth mechanisms of some electrodeposited CuCo granular films. J Magn Magn Mater 224:221–232. https://doi.org/10.1016/s0304-8853(01)00038-5
Kuang M, Han P, Wang Q et al (2016) CuCo hybrid oxides as bifunctional electrocatalyst for efficient water splitting. Adv Funct Mater 26:8555–8561. https://doi.org/10.1002/adfm.201604804
Yang Y, Qi X, Wang X et al (2016) Deactivation study of CuCo catalyst for higher alcohol synthesis via syngas. Catal Today 270:101–107. https://doi.org/10.1016/j.cattod.2015.06.014
Allia P, Coisson M, Tiberto P et al (1999) Magnetic hysteresis in granular CuCo alloys. J Appl Phys 85:4343. https://doi.org/10.1063/1.370362
Mader S, Widmer H, d’Heurle FM, Nowick AS (1963) Metastable alloys of CuCo and CuAg thin films deposited in vacuum. Appl Phys Lett 3:201–203. https://doi.org/10.1063/1.1753848
Tavares Figueiredo R, López Granados M, Fierro JLG et al (1998) Preparation of alumina-supported CuCo catalysts from cyanide complexes and their performance in CO hydrogenation. Appl Catal A Gen 170:145–157. https://doi.org/10.1016/s0926-860x(98)00037-4
Fedosyuk VM, Schwarzacher W, Kasyutich OI, Tochitsky TA (1998) Granular CuCo nanowires. Metallofiz i Noveishie Tekhnologii 20:65–70
Muzikansky A, Nanikashvili P, Grinblat J, Zitoun D (2013) Ag dewetting in Cu@Ag monodisperse core–shell nanoparticles. J Phys Chem C 117:3093–3100. https://doi.org/10.1021/jp3109545
Rocha AL, Solórzano IG, Vander Sande JB (2007) Heterogeneous and homogeneous nanoscale precipitation in dilute Cu–Co alloys. Mater Sci Eng C 27:1215–1221. https://doi.org/10.1016/j.msec.2006.08.032
Suehiro K, Nishimura S, Horita Z et al (2008) High-pressure torsion for production of magnetoresistance in Cu–Co alloy. J Mater Sci 43:7349–7353. https://doi.org/10.1007/s10853-008-2813-9
Miranda MGM, Estévez-Rams E, Martínez G, Baibich MN (2003) Phase separation in Cu90Co10 high-magnetoresistance materials. Phys Rev B 68:014434. https://doi.org/10.1103/physrevb.68.014434
Berkowitz AE, Mitchell JR, Carey MJ et al (1992) Giant magnetoresistance in heterogeneous Cu–Co alloys. Phys Rev Lett 68:3745–3748. https://doi.org/10.1103/physrevlett.68.3745
Childress JR, Chien CL (1991) Reentrant magnetic behavior in fcc Co–Cu alloys. Phys Rev B 43:8089–8093. https://doi.org/10.1103/physrevb.43.8089
Gangopadhyay S, Hadjipanayis GC, Sorensen CM, Klabunde KJ (1992) Magnetic properties of ultrafine Co particles. IEEE Trans Magn 28:3174–3176. https://doi.org/10.1109/20.179749
Yu RH, Zhang XX, Tejada J et al (1996) Structure, magnetic properties, and giant magnetoresistance in melt-spun metallic copper–cobalt ribbons. J Appl Phys 79:1979–1990. https://doi.org/10.1063/1.361049
I.H.Karahan OFBMB (2007) Giant magnetoresistance of electrodeposited Cu–Co–Ni alloy films. Pramana J Phys 68:83–90
Dang J, Chou KC, Hu XJ, Zhang GH (2013) Reduction kinetics of metal oxides by hydrogen. Steel Res Int 84:526–533. https://doi.org/10.1002/srin.201200242
Hickey BJ, Howson MA, Musa SO, Wiser N (1995) Giant magnetoresistance for superparamagnetic particles: melt-spun granular CuCo. Phys Rev B 51:667–669. https://doi.org/10.1103/physrevb.51.667
Fedosyuk VM, Kasyutich OI, Ravinder D, Blythe HJ (1996) Giant magnetoresistance in granular electrodeposited CuCo films. J Magn Magn Mater 156:345–346. https://doi.org/10.1016/0304-8853(95)00893-4
Allia P, Baricco M, Knobel M et al (1995) Giant magnetoresistance in Joule heated CuCo ribbons. J Magn Magn Mater 140–144:617–618. https://doi.org/10.1016/0304-8853(94)01514-7
Lin Z, Zhan-guo F (2009) Giant magnetoresistance and microstructure in CuCo granular flims prepared by electrodeposition. In: The materials society annual meeting, pp 19–26
Turgut Z, Horwath JC, Fingers RT (2008) Powder metallurgy processing of high-strength FeCo alloys. Power Div (preprint)
Parhi BR, Sahoo SK, Mishra SC et al (2016) Upgradation of bauxite by molecular hydrogen and hydrogen plasma. Int J Miner Metall Mater 23:1141–1149. https://doi.org/10.1007/s12613-016-1333-x
Parhi BR, Sahoo SK, Sahu M et al (2017) Physico-chemical investigations of high iron bauxite for application of refractive and ceramics. Metall Res Technol 114:307. https://doi.org/10.1051/metal/2017025
Moustafa AF (2017) Isothermal reduction process and kinetic of nanomaterials in reducing atmosphere: a review. J Anal Appl Pyrolysis 127:126–139. https://doi.org/10.1016/j.jaap.2017.08.015
Luo SD, Qian M (2018) Microwave processing of titanium and titanium alloys for structural, biomedical and shape memory applications: current status and challenges. Mater Manuf Process 33:35–49. https://doi.org/10.1080/10426914.2016.1257800
Furuyama K, Yamanaka K, Higurashi E, Suga T (2018) Evaluation of hydrogen radical treatment for indium surface oxide removal and analysis of re-oxidation behavior. Jpn J Appl Phys 57:02BC01. https://doi.org/10.7567/jjap.57.02bc01
Di L, Zhang J, Zhang X (2018) A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process Polym 15:1700234. https://doi.org/10.1002/ppap.201700234
Römermann H, Müller A, Bomhardt K et al (2018) Formation of metal (nano-)particles in drying latex films by means of a reducing plasma: a route to auto-stratification. J Phys D Appl Phys 51:215205. https://doi.org/10.1088/1361-6463/aabf2c
Sener ME, Caruana DJ (2018) Modulation of copper(I) oxide reduction/oxidation in atmospheric pressure plasma jet. Electrochem Commun 95:38–42. https://doi.org/10.1016/j.elecom.2018.08.014
Fedorovich OA, Hladkovskyi VV, Polozov BP et al (2018) Peculiarities of interaction of low-energy protons with tungsten surface. Probl At Sci Technol 116:302–306
Tennyson J, Rahimi S, Hill C et al (2017) QDB: a new database of plasma chemistries and reactions. Plasma Sources Sci, Technol, p 26
Parhi BR, Sahoo SK, Bhoi B et al (2016) Application of hydrogen for the reduction of bauxite mineral. In: IOP conference series: materials science and engineering
Mandal AK, Dishwar RK, Sinha OP (2018) Behavior of an indigenously fabricated transferred arc plasma furnace for smelting studies. Plasma Sci Technol 20:035506. https://doi.org/10.1088/2058-6272/aa9cde
Ji G, Smart S, Bhatia SK, Diniz da Costa JC (2015) Improved pore connectivity by the reduction of cobalt oxide silica membranes. Sep Purif Technol 154:338–344. https://doi.org/10.1016/j.seppur.2015.09.065
Mandal AK, Dishwar RK, Sinha OP (2018) Design, fabrication, and characterization of an indigenously fabricated prototype transferred arc plasma furnace. IEEE Trans Plasma Sci 46:1793–1799. https://doi.org/10.1109/tps.2018.2817234
Elg DT, Panici GA, Liu S et al (2018) Removal of tin from extreme ultraviolet collector optics by in-situ hydrogen plasma etching. Plasma Chem Plasma Process 38:223–245. https://doi.org/10.1007/s11090-017-9852-4
Altmannshofer S, Eisele I, Gschwandtner A (2016) Hydrogen microwave plasma treatment of Si and SiO2. Surf Coat Technol 304:359–363. https://doi.org/10.1016/j.surfcoat.2016.07.038
Vesel A, Drenik A, Mozeti M (2013) Removing of oxides from Fe–Ni alloys by hydrogen plasma treatment. In: International conference nuclear energy for new Europe 2007, pp 1–4
Mozetič M, Vesel A, Kovač J et al (2015) Formation and reduction of thin oxide films on a stainless steel surface upon subsequent treatments with oxygen and hydrogen plasma. Thin Solid Films 591:186–193. https://doi.org/10.1016/j.tsf.2015.02.007
Vesel A, Mozetič M, Balat-Pichelin M (2015) Sequential oxidation and reduction of tungsten/tungsten oxide. Thin Solid Films 591:174–181. https://doi.org/10.1016/j.tsf.2015.02.019
Vesel A, Mozetic M, Balat-Pichelin M (2016) Reduction of a thin chromium oxide film on Inconel surface upon treatment with hydrogen plasma. Appl Surf Sci 387:1140–1146. https://doi.org/10.1016/j.apsusc.2016.06.098
Badin V, Diamanti E, Forêt P et al (2015) Design of stainless steel porous surfaces by oxide reduction with hydrogen. Mater Des 86:765–770. https://doi.org/10.1016/j.matdes.2015.07.142
Pradhan SK, Jeevitha M, Singh SK (2015) Plasma cleaning of old Indian coin in H2–Ar atmosphere. Appl Surf Sci 357:445–451. https://doi.org/10.1016/j.apsusc.2015.09.026
Lee JH, Kang DS, Moon MK, Hong SK (2016) Separating technology of pure zirconia from zircon-sand by the Ar–H2 arc plasma fusion and the microwave leaching. Mater Sci Forum 879:1080–1085. https://doi.org/10.4028/www.scientific.net/MSF.879.1080
Sabat KC, Rajput P, Paramguru RK et al (2014) Reduction of oxide minerals by hydrogen plasma: an overview. Plasma Chem Plasma Process 34:1–23. https://doi.org/10.1007/s11090-013-9484-2
Rajput P, Sabat KC, Paramguru RK et al (2014) Direct reduction of iron in low temperature hydrogen plasma. Ironmak Steelmak 41:721–731. https://doi.org/10.1179/1743281214y.0000000186
Sabat KC, Paramguru RK, Pradhan S, Mishra BK (2015) Reduction of cobalt oxide (Co3O4) by low temperature hydrogen plasma. Plasma Chem Plasma Process 35:387–399. https://doi.org/10.1007/s11090-014-9602-9
Sabat KC, Paramguru RK, Mishra BK (2016) Reduction of copper oxide by low-temperature hydrogen plasma. Plasma Chem Plasma Process 36:1111–1124. https://doi.org/10.1007/s11090-016-9710-9
Sabat KC, Paramguru RK, Mishra BK (2017) Reduction of oxide mixtures of (Fe2O3 + CuO) and (Fe2O3 + Co3O4) by low-temperature hydrogen plasma. Plasma Chem Plasma Process 37:979–995. https://doi.org/10.1007/s11090-017-9818-6
Sabat KC, Murphy AB (2017) Hydrogen plasma processing of iron ore. Metall Mater Trans B 48:1561–1594. https://doi.org/10.1007/s11663-017-0957-1
Sabat KC, Paramguru RK, Mishra BK (2018) Formation of copper–nickel alloy from their oxide mixtures through reduction by low-temperature hydrogen plasma. Plasma Chem Plasma Process 38:621–635. https://doi.org/10.1007/s11090-018-9880-8
ASM International (2004) Alloy phase diagrams, vol 3. ASM handbook. ASM International, Russell Township
Massalski TB, Okamoto H, Subramanian PR, Kacprzak L (1990) Binary alloy phase diagrams, 2nd edn. ASM International, Materials Park, OH, ISBN: 978-0-87170-403-0
Nishizawa T, Ishida K (1984) The Co–Cu (Cobalt–Copper) system. Bull Alloys Phase Diagr 5:161–165. https://doi.org/10.1007/bf02868953
Straumal BB, Kilmametov AR, Ivanisenko Y et al (2014) Phase transitions during high pressure torsion of CuCo alloys. Mater Lett 118:111–114. https://doi.org/10.1016/j.matlet.2013.12.042
Bachmaier A, Aboulfadl H, Pfaff M et al (2015) Structural evolution and strain induced mixing in Cu–Co composites studied by transmission electron microscopy and atom probe tomography. Mater Charact 100:178–191. https://doi.org/10.1016/j.matchar.2014.12.022
Kormout KS, Pippan R, Bachmaier A (2017) Deformation-induced supersaturation in immiscible material systems during high-pressure torsion. Adv Eng Mater 19:1–19. https://doi.org/10.1002/adem.201600675
Gente C, Oehring M, Bormann R (1993) Formation of thermodynamically unstable solid solutions in the Cu–Co system by mechanical alloying. Phys Rev B 48:13244–13252. https://doi.org/10.1103/physrevb.48.13244
Froes FH, Senkov ON, Baburaj EG (2001) Synthesis of nanocrystalline materials—an overview. Mater Sci Eng A 301:44–53. https://doi.org/10.1016/s0921-5093(00)01391-5
Busch R, Gärtner F, Borchers C et al (1996) High resolution microstructure analysis of the decomposition of Cu90Co10 alloys. Acta Mater 44:2567–2579. https://doi.org/10.1016/1359-6454(95)00370-3
Kubaschewski O, Alcock CB (1979) Metallurgical thermochemistry. Pergamon Press, Oxford
Chase M (1998) NIST–JANAF thermochemical tables. In: Journal of Physical and Chemical Reference Data, Monograph, 4th edn, vol 9, p 1952
Ragone DV (1995) Thermodynamics of materials, vol 1. Wiley, Hoboken
Hassouni K, Gicquel A, Capitelli M, Loureiro J (1999) Chemical kinetics and energy transfer in moderate pressure H2 plasmas used in diamond MPACVD processes. Plasma Sources Sci Technol 8:494–512. https://doi.org/10.1088/0963-0252/8/3/320
Cullity SR, Stock BD (2001) Elements of X-ray diffraction. Prentice Hall, Upper Saddle River
Suryanarayana C, Norton MG (1998) X-rays and diffraction. Springer, Boston
Bindu P, Thomas S (2014) Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J Theor Appl Phys 8:123–134. https://doi.org/10.1007/s40094-014-0141-9
Rodriguez JA, Kim JY, Hanson JC et al (2003) Reduction of CuO in H2: in situ time-resolved XRD studies. Catal Lett 85:247–254. https://doi.org/10.1023/a:1022110200942
Kim JY, Rodriguez A, Hanson JC et al (2003) Reduction of CuO and Cu2O with H2: H embedding and kinetic effects in the formation of suboxides. J Am Chem Soc 125(35):10684–10692
Wang H, Huang Y, Tan Z, Hu X (2004) Fabrication and characterization of copper nanoparticle thin-films and the electrocatalytic behavior. Anal Chim Acta 526:13–17. https://doi.org/10.1016/j.aca.2004.08.060
Gupta S, Gartley M (1999) XRD and VSM analysis of nanostructured Cu–Co alloys. JCPDS—International Centre Diffraction Data, pp 688–697. http://www.icdd.com/resources/axa/vol41/v41_75.pdf
Piotrowski K, Mondal K, Wiltowski T et al (2007) Topochemical approach of kinetics of the reduction of hematite to wustite. Chem Eng J 131:73–82. https://doi.org/10.1016/j.cej.2006.12.024
Wang H, Sohn HY (2013) Hydrogen reduction kinetics of magnetite concentrate particles relevant to a novel flash ironmaking process. Metall Mater Trans B Process Metall Mater Process Sci 44:133–145. https://doi.org/10.1007/s11663-012-9754-z
Chen F, Mohassab Y, Jiang T, Sohn HY (2015) Hydrogen reduction kinetics of hematite concentrate particles relevant to a novel flash ironmaking process. Metall Mater Trans B 46:1133–1145. https://doi.org/10.1007/s11663-015-0332-z
Yang X, Zhang Y (2012) Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater Chem Phys 132:233–238. https://doi.org/10.1016/j.matchemphys.2011.11.021
Jacob KT, Raj S, Rannesh L (2007) Vegard’s law: a fundamental relation or an approximation? Int J Mater Res 98:776–779. https://doi.org/10.3139/146.101545
King HW (1966) Quantitative size-factors for metallic solid solutions. J Mater Sci 1:79–90. https://doi.org/10.1007/bf00549722
Lubarda VA (2003) On the effective lattice parameter of binary alloys. Mech Mater 35:53–68
Turchanin MA, Agraval PG (2007) Phase equilibria and thermodynamics of binary copper systems with 3d-metals. V. Copper-cobalt system. Powder Metall Met Ceram 46:77–89. https://doi.org/10.1007/s11106-007-0013-9
Gaskell DR, Laughlin DE (2018) Introduction to the thermodynamics of materials, 6th edn. CRC Press, New York
Acknowledgements
I am thankful to Prof. (Dr.) Barada Kanta Mishra, Director, Indian Institute of Technology Goa, India, and Prof. (Dr.) Raja Kishore Paramguru, Professor in School of Mechanical Engineering, KIIT University, Bhubaneswar, India, for their constructive technical advice. I would also like to thank CSIR, New Delhi for providing financial support to carry out research work under the project MINMET, Project No. ESC 205. I am immensely grateful to the reviewers for their so-called insights.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sabat, K.C. Formation of CuCo Alloy From Their Oxide Mixtures Through Reduction by Low-Temperature Hydrogen Plasma. Plasma Chem Plasma Process 39, 1071–1086 (2019). https://doi.org/10.1007/s11090-019-09963-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-09963-y