Abstract
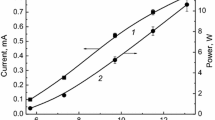
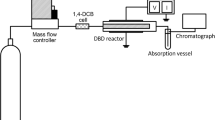
The processes of degradation of 2,4-dichlorophenol (2,4-DCP) under the action of atmospheric pressure of dielectric barrier discharge (DBD) in oxygen were studied. It was shown that the degradation of 2,4-DCP proceeds efficiently. Degree of decomposition reaches 90%. The degradation kinetics of 2,4-DCP obeys the formal first-order kinetic law on concentration of 2,4-DCP. The effective rate constants depend weakly on the experimental conditions and are equal to ~0.2 s−1. Based on experimental data, the energy efficiency of decomposition of 2,4-DCP was determined. Depending on the conditions, the energy efficiency was in the range of (8–90) × 10−3 molecules per 100 eV. The composition of the products was studied by gas chromatography (GC), gas chromatography–mass spectrometry (GC–MS), energy-dispersive X-ray spectroscopy (EDX), attenuated total reflection-fourier transform infrared (ATR-FTIR) spectroscopy, electron spin resonance (ESR) spectroscopy and UV/Visible spectroscopy. It was shown that about ~20% of 2,4-DCP is converted to CO2, while the other part forms an organic film on the reactor wall. The substance formed is close to the carboxylic acids in chemical composition and exhibits electrical conductivity and paramagnetic properties. Almost all of the chlorine contained in the 2,4-DCP is released into the gas phase. The active species of the afterglow react with liquid hexane, forming the products of its oxidation. Some assumptions regarding the pathway of the process are discussed.









Similar content being viewed by others
References
Chen HL, Lee HM, Chen SH, Chang MB, Yu SJ, Li SN (2009) Removal of volatile organic compounds by single-stage and two-stage plasma catalysis systems: a review of the performance enhancement mechanisms, current status, and suitable applications. Environ Sci Technol 43(7):2216–2227
Sultana S, Vandenbroucke AM, Leys C, Geyter ND, Morent R (2015) Abatement of VOCs with alternate adsorption and plasma-assisted regeneration: a review. Catalysts 5(2):718–746
Thevenet F, Sivachandiran L, Guaitella O, Barakat C, Rousseau A (2014) Plasma–catalyst coupling for volatile organic compound removal and indoor air treatment: a review. J Phys D Appl Phys 47(22):224011
Whitehead JC (2010) Plasma catalysis: a solution for environmental problems. Pure Appl Chem 82(6):1329–1336
Kim H-H, Teramoto Y, Ogata A, Takagi H, Nanba T (2016) Plasma catalysis for environmental treatment and energy applications. Plasma Chem Plasma Process 36(1):45–72
Parkinson WH, Yoshino K, Freeman DE (1988) Absolute absorption cross section measurements of ozone and the temperature dependence at four reference wavelengths leading to renormalization of the cross section between 240 and 350 nm. Smithsonian Institution Astrophysical Observatory, MA, p 02138
Hillebrand WF, Lundell GEF (1953) Applied inorganic analysis, 2nd edn. Wiley, New York
Water Chlorine (Residual) No. 2 (1971) Analytical reference service, Rep. No. 40. U.S. Environmental Protection Agency, Cincinnati, Ohio
Smirnov SA, Shutov DA, Bobkova ES, Rybkin VV (2015) Physical parameters and chemical composition of a nitrogen DC discharge with water cathode. Plasma Chem Plasma Process 35(4):639–657
Kudryashov SV, Shchegoleva GS, Sirotkina EE, Ryabov AYu (2000) Oxidation of hydrocarbons in a barrier discharge reactor. High Energy Chem 34(2):112–115
Bresch S, Wandell R, Wang H, Alabugin I, Locke BR (2016) Oxidized derivatives of n-hexane from a water/argon continuous flow electrical discharge plasma reactor. Plasma Chem Plasma Process 36(2):553–584
Bubnov AG, Grinevich VI, Aleksandrova SN, Kostrov VV (1997) Polymerization of phenol vapor in a barrier-discharge plasma. High Energy Chem 31(4):268–274
Boganov SE, Kudryashov SV, Ryabov AY, Suslov AI, Rynin SS, Egorov MP, Nefedov OM (2014) Matrix IR study of benzene transformations in a pulsed glow discharge in the absence and the presence of oxygen. Plasma Chem Plasma Process 34(6):1345–1370
Shriner RL, Hermann CKF, Morrill TC, Curtin DY, Fuson RC (2004) The systematic identification of organic compounds, 8th edn. Wiley
Bellamy LJ (1980) The infra-red spectra of complex molecules. Chapman and Hall, London and New York
Reid RC, Prausnitz JM, Sherwood TK (1977) The properties of gases and liquids. McGraw-Hill, New York. doi:10.1002/aic.690240634
Bobkova ES, Khodor YV, Kornilova ON, Rybkin VV (2014) Chemical composition of plasma of dielectric barrier discharge at atmospheric pressure with a liquid electrode. High Temp 52(4):511–517
Bobkova ES, Rybkin VV (2013) Estimation of electron parameters in the dielectric barrier discharge with a liquid electrode at atmospheric pressure. High Temp 51(6):747–752
Eliasson B, Hirth M, Kogelschatz U (1987) Ozone synthesis from oxygen in dielectric barrier discharges. J Phys D Appl Phys 20(11):1421–1437
Dorofeev YUI (2004) Gaseous products of polyethylene etching by O(3 P) atoms involving O2(X 3 Σ − g ), O2(b 1 Σ + g ), and O2(a 1Δ g ) oxygen molecules and a possible mechanism of their formation. High Energy Chem 38(4):221–225
Xu F, Wang H, Zhang QZ, Zhang RX, Qu XH, Wang WX (2010) Kinetic properties for the complete series reactions of chlorophenols with OH radicals—relevance for dioxin formation. Environ Sci Technol 44(4):1399–1404
Evans CS, Dellinger B (2005) Mechanisms of dioxin formation from the high-temperature oxidation of 2-chlorophenol. Environ Sci Technol 39(1):22–127
Altarawneh M, Dlugogorski BZ, Kennedy EM, Mackie JC (2008) Quantum chemical and kinetic study of formation of 2-chlorophenoxy radical from 2-chlorophenol: unimolecular decomposition and bimolecular reactions with H, OH, Cl, and O2. J Phys Chem 112(16):3680–3692
Kilic M, Kocturk G, San N, Cinar Z (2007) A model for prediction of product distributions for the reactions of phenol derivatives with hydroxyl radicals. Chemosphere 69(9):1396–1408
Acknowledgements
This study was carried out in the frame of Project part of State Assignment of the Ministry of Education and Science of the RF, No 3.1371.2017/4.6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gushchin, A.A., Grinevich, V.I., Kozlov, A.A. et al. Destruction of 2,4 Dichlorophenol in an Atmospheric Pressure Dielectric Barrier Discharge in Oxygen. Plasma Chem Plasma Process 37, 1331–1341 (2017). https://doi.org/10.1007/s11090-017-9828-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9828-4