Abstract
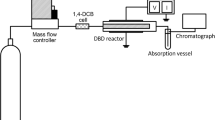
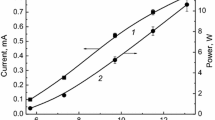
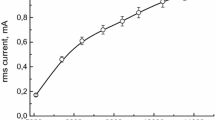
The kinetics of decomposition of 1,4-dichlorobenzene vapor in an atmospheric pressure dielectric barrier discharge in oxygen has been studied. It has been found that the degree of decomposition decreased with the gas flow rate and initial concentration and increased with the discharge power. The apparent rate constants of degradation have been determined, and the energy efficiency of the process has been estimated. The maximum degree of decomposition reached 90%, and the energy yield of decomposition was 2.7 × 10–3 molecules per 100 eV. The main gaseous products of the degradation were СО2, Сl2, carboxylic acids, and aldehydes. The degradation was accompanied by the formation of a polymer film on the reactor walls. Elemental analysis has shown that the film contains C (60 wt %), O (38%), and Cl (2.3%). The IR spectra show that the film contains fragments of carboxylic acids. A comparison of the experimental results with previously obtained data for 2,4-dichlorophenol has shown that the kinetic laws are similar for both of the compounds.



Similar content being viewed by others
REFERENCES
Boeglin, M.L., Wessels, D., and Henshel, D., Environ. Res., 2006, vol. 100, no. 2, p. 242.
Huang, B., Lei, C., Wei, C., and Zeng, G., Environ. Int., 2014, vol. 71, p. 118.
Sultana, S., Vandenbroucke, A.M., Leys, C., Geyter, N.D., and Morent, R., Catalysts, 2015, vol. 5, no. 2, p. 718.
Thevenet, F., Sivachandiran, L., Guaitella, O., Barakat, C., and Rousseau, A., J. Phys. D: Appl. Phys., 2014, vol. 47, no. 22, p. 224011.
Kim, H.-H., Teramoto, Y., Ogata, A., Takagi, H., and Nanba, T., Plasma Chem. Plasma Process., 2016, vol. 36, no. 1, p. 45.
Krasnoperov, L.N., Krishtova, L.G., Joseph, W., and Bozzelli, J.W., J. Adv. Oxid. Technol., 1997, vol. 2, no. 1, p. 248.
Indarto, A., Environ. Technol., 2011, vol. 33, nos. 4–6, p. 663.
Marotta, E., Scorrano, G., and Paradisi, C., Plasma Process. Polym., 2005, vol. 2, no. 3, p. 209.
Gushchin, A.A., Grinevich, V.I., Kozlov, A.A., Kvitkova, E.Yu., Shutov, D.A., and Rybkin, V.V., Plasma Chem. Plasma Process., 2017, vol. 37, no. 5, p. 1331.
Gushchin, A.A., Grinevich, V.I., Shulyk, V.Ya., Kvitkova, E.Yu., and Rybkin, V.V., Plasma Chem. Plasma Process., 2018, vol. 38, no. 1, p. 123.
Bobkova, E.S. and Rybkin, V.V., Plasma Chem. Plasma Process., 2015, vol. 35, no. 1, p. 133.
Malik, M.A., Plasma Chem. Plasma Process., 2010, vol. 30, no. 1, p. 21.
GOST R (Russian State Standard) 55227-2012: Water: Methods for Determination of Formaldehyde Content, Moscow: Standartinform, 2008.
Simonov, V.A., Nekhorosheva, E.V., and Zavorovskaya, N.A., Analiz vozdushnoi sredy pri pererabotke polimernykh materialov (Analysis of the Air Medium during Processing of Polymer Materials), Leningrad: Khimiya, 1988.
Bobkova, E.S., Khodor, Yu.V., Kornilova, O.N., and Rybkin, V.V., High Temp, 2014, vol. 52, no. 4, p. 511.
Bellamy, L.J., The Infrared Spectra of Complex Molecules, London: Chapman and Hall, 1980.
Bubnov, A.G., Grinevich, V.I., Aleksandrova, S.N., and Kostrov, V.V., High Energy Chem., 1997, vol. 31, no. 4, p. 268.
Boganov, S.E., Kudryashov, S.V., Ryabov, A.Yu., Suslov, A.I., Rynin, S.S., Egorov, M.P., and Nefedov, O.M., Plasma Chem. Plasma Process., 2014, vol. 34, no. 6, p. 1345.
Yasuda, H.K., Plasma Polymerization, New York: Academic, 1985.
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation, project no. 3.1371.2017/4.6, and the Russian Foundation for Basic Research, grant no. 18-08-01239 A.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Gushchin, A.A., Grinevich, V.I., Kozlov, A.A. et al. Kinetics of 1,4-Dichlorobenzene Decomposition in an Atmospheric Pressure Dielectric Barrier Discharge in Oxygen. High Energy Chem 54, 64–68 (2020). https://doi.org/10.1134/S0018143920010063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143920010063