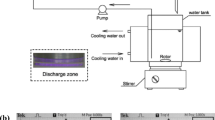
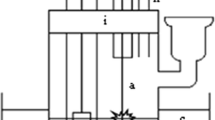
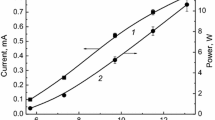
Abstract
In the present study, efficient dechlorination and decomposition of dichloromethane (DCM) induced by glow discharge plasma (GDP) in contact with an aqueous solution was investigated. Experimental results showed that DCM underwent effective dechlorination and decomposition under the action of GDP. Both the removal and the dechlorination of DCM increased with increasing pH and with the presence of hydroxyl radical scavengers and decreased with quenchers of hydrated electrons. Formic acid and formaldehyde were the major intermediate byproducts. Final products were carbon dioxide and chloride ion. Hydrated electrons were the most important active species responsible for initiation of the reaction. Hydrolysis of the resulting chloromethyl radicals played an important role in mineralization of chlorine atoms of the molecule. Hydroxyl radicals were mainly involved in the oxidation of the intermediate byproducts. Reaction mechanism was proposed based on the dechlorination kinetics and the distribution of intermediate byproducts.








Similar content being viewed by others
References
Rossberg M, Lendle W, Pfleiderer G, Tögel A, Torkelson T, Beutel K (2011) Chloromethanes, Ullmann’s encyclopedia of industrial chemistry. Wiley, Weinheim
Cooper G, Bale A, Schlosser P (2011) Toxicological review of dichloromethane. US EPA. http://www.epa.gov/iris/toxreviews/0070tr.pdf
Fagin J, Bradley J, Williams D (1980) Carbon monoxide poisoning secondary to inhaling methylene chloride. Br Med J 281:1461
WHO (1999) Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. IARC Monogr Eval Carcinog Risks Hum 71:251–252
Dill DC, Murphy PG, Mayes MA (1987) Toxicity of methylene chloride to life stages of the fathead minnow, Pimephales promelas Rafinesque. Bull Environ Contam Toxicol 39:869–876
Shestakova M, Sillanpää M (2013) Removal of dichloromethane from ground and wastewater: a review. Chemosphere 93(7):1258–1267
Hsiao CY, Lee CL, Ollis DF (1983) Heterogeneous photocatalysis: degradation of dilute solutions of dichloromethane (CH2Cl2), chloroform (CHCl3), and carbon tetrachloride (CCl4) with illuminated TiO2 photocatalyst. J Catal 82:418–423
Hua I, Hoffmann MR (1996) Kinetics and mechanism of the sonolytic degradation of CCl4: intermediates and byproducts. Environ Sci Technol 30:864–871
Getoff N (1986) Radiation induced decomposition of some chlorinated methanes in water. Water Res 20:1261–1264
Susanta KS, Rajeshwar S, Ashok KS (1998) A study on the origin of non-Faradaic behavior of anodic contact glow discharge electrolysis. J Electrochem Soc 145:2209–2213
Liu Y, Sun B, Wang L, Wang D (2012) Characteristics of light emission and radicals formed by contact glow discharge electrolysis of an aqueous solution. Plasma Chem Plasma Process 32:359–368
Bullock AT, Gavin DL, Ingram MD (1980) Electron spin resonance detection of spin-trapped radicals formed during the glow-discharge electrolysis of aqueous solutions. J Chem Soc Faraday Trans 76:648–653
Goodman J, Hickling A, Schofield B (1973) The yield of hydrated electrons in glow-discharge electrolysis. J Electroanal Chem Interfacial Electrochem 48:319–322
Tezuka M, Iwasaki M (1998) Plasma induced degradation of chlorophenols in an aqueous solution. Thin Solid Films 316:123–126
Gao J, Liu Y, Yang W, Pu L, Yu J, Lu Q (2003) Oxidative degradation of phenol in aqueous electrolyte induced by plasma from a direct glow discharge. Plasma Sources Sci Technol 12:533–538
Gai K, Dong Y (2005) Liquid phase auramine oxidation induced by plasma with glow discharge electrolysis. Plasma Sources Sci Technol 14:589–593
Wang L, Jiang X, Liu Y (2007) Efficient degradation of nitrobenzene induced by glow discharge plasma in aqueous solution. Plasma Chem Plasma Process 27:504–515
Gai K (2007) Plasma-induced degradation of diphenylamine in aqueous solution. J Hazard Mater 146:249–254
Jin X, Zhang H, Wang X, Zhou M (2012) An improved multi-anode contact glow discharge electrolysis reactor for dye discoloration. Electrochim Acta 59:474–478
Wang L, Liu Y (2012) Enhancement of phenol degradation by electron acceptors in anodic contact glow discharge electrolysis. Plasma Chem Plasma Process 32:715–722
Wang L, Zeng H, Xin Y (2014) Dechlorination and decomposition of trichloroacetic acid by glow discharge plasma in aqueous solution. Electrochim Acta 115:332–336
Wang L, Liu P, Zhang S (2015) Debromination and decomposition of bromoform by contact glow discharge electrolysis in an aqueous solution. Electrochim Acta 165:390–395
Fung K, Grosjean D (1981) Determination of nanogram amounts of carbonyls as 2, 4-dinitrophenylhydrazones by high-performance liquid chromatography. Anal Chem 53:168–171
Balkas TI (1972) The radiolysis of aqueous solutions of methylene chloride. Int J Radiat Phys Chem 4:199–208
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals. Phys Chem Ref Data 17:513–886
Von Sonntag C (2006) Peroxyl radicals, free-radical-induced DNA damage and its repair: a chemical perspective. Springer, Berlin, pp 159–194
Halmann M, Hunt AJ, Spath D (1992) Photodegradation of dichloromethane, tetrachloroethylene and 1, 2-dibromo-3-chloropropane in aqueous suspensions of TiO2 with natural, concentrated and simulated sunlight. Sol Energy Mater Sol Cells 26:1–16
Acknowledgments
This work was supported by the National Science Foundation of China (51008262) and the Natural Science Foundation of Fujian province, China (2015J01651).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Liu, P. & Chen, T. Glow Discharge Plasma Induced Dechlorination and Decomposition of Dichloromethane in an Aqueous Solution. Plasma Chem Plasma Process 36, 615–626 (2016). https://doi.org/10.1007/s11090-015-9658-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-015-9658-1