Abstract
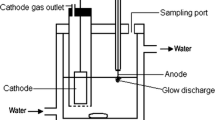
Anodic contact glow discharge electrolysis (CGDE) is a DC-excited atmospheric pressure discharge, in which a steady non-thermal plasma is generated locally between the surface of an electrolytic solution and an anode in contact with it. The I–U characteristics of CGDE were investigated. The plasma temperatures were estimated to be in the range, 1373–2045 K. Hydroxyl radicals and hydrogen peroxide were the main oxidants generated by CGDE. The hydrogen peroxide concentration reached 31.2 mmol/L (mM) in a phosphate buffer solution without organic substrates. During CGDE, the DFPs and the corresponding total organic carbon (TOC) in water were consumed. Most of the fluorine atoms in the DFPs were converted to fluoride ions, and the fluoride concentration increased steadily. An analysis of the hydroxylation of DFPs suggested that the hydroxyl radicals generated by CGDE were the key species responsible for the degradation of DFPs, and the possible mechanistic routes of the mineralization of DFPs are proposed. The disappearance of DFPs and the TOC as well as the defluorination of the DFPs followed first-order kinetics. The rate of TOC disappearance was relatively constant: 1.00 ± 0.05 × 10−2 min−1. The order of disappearance of the DFPs was 2,6-DFP > 2,3-DFP > 2,5-DFP > 2,4-DFP > 3,4-DFP > 3,5-DFP. In contrast, the order of defluorination of the DFPs was 2,5-DFP > 2,3-DFP > 2,6-DFP > 2,4-DFP > 3,4-DFP > 3,5-DFP. Overall, the order of the reaction rates for each DFP was kDFP > kdF > kTOC.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.















Similar content being viewed by others
References
Shoute LCT, Mittal JP (1996) J Phys Chem 100:3016
Liu YJ, Jiang XZ (2008) Plasma Chem Plasma Process 28(1):15
Joshi RP, Thagard SM (2013) Plasma Chem Plasma Process 33(1):17
Zeng MD, Zhao K, Lu Y, Ou YJ, Liu DQ, Wang M, Ma YM (2015) Plasma Chem Plasma Process 35(4):721
Hao XL, Zhou MH, Zhang Y, Lei LC (2006) Plasma Chem Plasma Process 26(5):455
Wen YZ, Jiang XZ, Liu WP (2002) Plasma Chem Plasma Process 22(1):175
Du CM, Yan JH, Cheron BG (2007) Plasma Chem Plasma Process 27(5):635
Dayal AR, Pfluger D, Kearney TN, Western RJ, McAllister T (2004) Plasma Chem Plasma Process 24(4):573
Key BD, Howell RD, Criddle CS (1997) Environ Sci Technol 31:2445
Ravichandran L, Selvam K, Swaminathan M (2007) Aust J Chem 60:951
Ferreira MIM, Marchesi JR, Janssen DB (2008) Appl Microbiol Biotechnol 78:709
Franco AR, Ferreira AC, Castro PML (2014) Chemosphere 111:260
Goskonda S, Catallo WJ, Junk T (2002) Waste Manag 22:351
Tzedakis T, Savall A, Clifton MJ (1989) J Appl Electochem 19:911
Liu YJ (2009) J Hazard Mater 166:1495
Yang HM, Matsumoto Y, Tezuka M (2009) J Environ Sci (Suppl 1):142
Yang HM, Tezuka M (2011) J Phys D Appl Phys 44:155203
Yang HM, Tezuka M (2011) J Environ Sci 23:1044
Hickling A, Ingram MD (1964) Trans Faraday Soc 60:783
Hickling A (1971) In: Bockris JOM, Conway BE (eds) Modern aspects of electrochemistry, vol 6. Butterworths, London, p 329
Bruggeman P, Leys C (2009) J Phys D Appl Phys 42:053001
Bruggeman P, Schram D, Rego R, Kong MG, Leys C (2009) Plasma Sources Sci Technol 18:025017
Gangal U, Srivastava M, Sen Gupta SK (2010) Plasma Chem Plasma Process 30(2):299
Gaisin AR, Son EE (2005) High Temp 43:1
Chen Q, Saito K, Takemura Y, Shirai H (2008) Thin Solid Films 516:6688
Yang HM, An BG, Wang SY, Li LX, Jin WJ, Li LH (2013) J Environ Sci 25(6):1
Yang HM, Cai X, Tezuka M (2013) Plasma Chem Plasma Process 33:1043
Wang L, Jiang XZ, Liu YJ (2008) J Hazard Mater 154:1106
Jin XL, Wang XY, Zhang HM, Xia Q, Wei DB, Yue JJ (2010) Plasma Chem Plasma Process 30:429
Wang XY, Zhou MH, Jin XL (2012) Electrochim Acta 80:501
Gong JY, Wang J, Xie WJ, Cai WM (2008) J Appl Electrochem 38:1749
Liu YJ (2009) J Hazard Mater 166:1495
Gao J, Wang X, Hu Z, Deng H, Hou J, Lu X, Kang J (2003) Water Res 37:267
Tomizawa S, Tezuka M (2006) Plasma Chem Plasma Process 26(1):43
Minero C, Aliberti C (1991) Langmuir 7:928
Aleshina GR, Sokolskaya NN, Sukhina OG (1979) Khim Prom-St Ser Reakt Osobo Chist Ves chestva 3:5
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51308276), Scientific Research Foundation for Doctors of Liaoning Province (Grant No. 20141123), Growth Plan for Distinguished Young Scholars in Colleges and Universities of Liaoning Province China (LJQ2015055), Anshan Science and Technology Program Project (Grant No. 2961), the National Natural Science Foundation of China (51102126), Innovative Research Team in Colleges and Universities of Liaoning Province China (LT2014007), Natural Science Foundation of Liaoning Province, China (2015020634).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, H., Zhao, X., Mengen, G. et al. Defluorination and Mineralization of Difluorophenols in Water by Anodic Contact Glow Discharge Electrolysis. Plasma Chem Plasma Process 36, 993–1009 (2016). https://doi.org/10.1007/s11090-016-9715-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9715-4