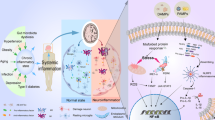
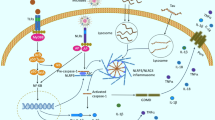
Abstract
Neuroinflammation is an inflammatory process in the central nervous system (CNS), in addition to being one of the main features of Alzheimer's disease (AD) and Parkinson's disease (PD). Microglia are known for their immune functions and have multiple reactive phenotypes related to the types of stages involving neurodegenerative diseases. Depending on the state of activation of microglia in the CNS, it can be neuroprotective or neurotoxic. In this context, AD is a neurodegenerative and neuroinflammatory disease characterized by the deposition of beta-amyloid plaques, formation of fibrillar tangles of tau protein, and loss of neurons due to neurotoxic activation of microglia. However, PD is characterized by the loss of dopaminergic neurons in the substantia nigra and accumulation of alpha-synuclein in the cortical regions, spinal cord, and brain stem, which occurs by microglial activation, contributing to the neuroinflammatory process. In this aspect, the activation of microglia in both pathologies triggers high levels of inflammatory markers, such as interleukins, and causes the neuroinflammatory process of the diseases. Thus, physical exercise is pointed out as neuroprotective, as it can act to strengthen neurogenesis and reduce the inflammatory process. Therefore, the present review addresses the neuroprotective effect of microglia after different types of physical exercise protocols and evaluates the activity and effects of inflammatory and anti-inflammatory parameters and mechanisms of AD and PD. This review will discuss the anti-inflammatory effects of physical exercise through microglia activation with neuroprotective activity and the role of pro-and anti-inflammatory cytokines in AD and PD.





Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Glass CK et al (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934
Stephenson J et al (2018) Inflammation in CNS neurodegenerative diseases. Immunology 154(2):204–219
Hanisch UK (2013) Functional diversity of microglia—how heterogeneous are they to begin with? Front Cell Neurosci 7:65
Dugger BN, Dickson DW (2017) Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 9(7):a028035
Kempuraj D et al (2016) Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 1(1):1003
Carson MJ et al (2006) CNS immune privilege: hiding in plain sight. Immunol Rev 213:48–65
Dornelles GL et al (2020) Ellagic acid inhibits neuroinflammation and cognitive impairment induced by lipopolysaccharides. Neurochem Res 45(10):2456–2473
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394
Subhramanyam CS et al (2019) Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 94:112–120
Zheng T, Zhang Z (2021) Activated microglia facilitate the transmission of α-synuclein in Parkinson’s disease. Neurochem Int 148:105094
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19(8):987–991
Baufeld C et al (2018) Differential contribution of microglia and monocytes in neurodegenerative diseases. J Neural Transm (Vienna) 125(5):809–826
De la Rosa A et al (2020) Physical exercise in the prevention and treatment of Alzheimer’s disease. J Sport Health Sci 9(5):394–404
Ozben T, Ozben S (2019) Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin Biochem 72:87–89
Sun X, Jin L, Ling P (2012) Review of drugs for Alzheimer’s disease. Drug Discov Ther 6(6):285–290
Wang WY et al (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3(10):136
Newcombe EA et al (2018) Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflamm 15(1):276
Kam TI et al (2020) Microglia and astrocyte dysfunction in Parkinson’s disease. Neurobiol Dis 144:105028
Mosley RL et al (2012) Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med 2(1):a009381
Khan AU et al (2019) Awareness and current knowledge of Parkinson’s disease: a neurodegenerative disorder. Int J Neurosci 129(1):55–93
Ellingson LD, Zaman A, Stegemöller EL (2019) Sedentary behavior and quality of life in individuals with Parkinson’s disease. Neurorehabil Neural Repair 33(8):595–601
Hu Y et al (2019) Exercise reverses dysregulation of T-Cell-related function in blood leukocytes of patients with parkinson’s disease. Front Neurol 10:1389
Feng YS et al (2020) The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci 245:117345
Soch A et al (2016) Effects of exercise on adolescent and adult hypothalamic and hippocampal neuroinflammation. Hippocampus 26(11):1435–1446
Nagamatsu LS et al (2014) Exercise is medicine, for the body and the brain. Br J Sports Med 48(12):943–944
Palasz E et al (2019) Neuroplasticity and neuroprotective effect of treadmill training in the chronic mouse model of Parkinson’s disease. Neural Plast 2019:8215017
Yoo SZ et al (2019) Effects of acute exercise on mitochondrial function, dynamics, and mitophagy in rat cardiac and skeletal muscles. Int Neurourol J 23(Suppl 1):S22-31
Perry VH, Teeling J (2013) Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol 35(5):601–612
Ransohoff RM, El Khoury J (2015) Microglia in health and disease. Cold Spring Harb Perspect Biol 8(1):a020560
Pignataro P et al (2021) FNDC5/Irisin system in neuroinflammation and neurodegenerative diseases: update and novel perspective. Int J Mol Sci 22(4):1605
Walter L, Neumann H (2009) Role of microglia in neuronal degeneration and regeneration. Semin Immunopathol 31(4):513–525
Wolf SA, Boddeke HW, Kettenmann H (2017) Microglia in physiology and disease. Annu Rev Physiol 79:619–643
Gao X et al (2016) In vivo reprogramming reactive glia into iPSCs to produce new neurons in the cortex following traumatic brain injury. Sci Rep 6:22490
Badanjak K et al (2021) The contribution of microglia to neuroinflammation in Parkinson’s Disease. Int J Mol Sci 22(9):4676
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9(1):42
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194
Prinz M, Jung S, Priller J (2019) Microglia biology: one century of evolving concepts. Cell 179(2):292–311
Sierra A et al (2013) Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci 7:6
Fakhoury M (2018) Microglia and astrocytes in Alzheimer’s disease: implications for therapy. Curr Neuropharmacol 16(5):508–518
Hickman S et al (2018) Microglia in neurodegeneration. Nat Neurosci 21(10):1359–1369
Ho MS (2019) Microglia in Parkinson’s Disease. Adv Exp Med Biol 1175:335–353
Ros-Bernal F et al (2011) Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc Natl Acad Sci USA 108(16):6632–6637
Yun SP et al (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24(7):931–938
Mee-Inta O, Zhao ZW, Kuo YM (2019) Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 8(7):691
Bobinski F et al (2018) Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 159(3):437–450
Kelly ÁM (2018) Exercise-induced modulation of neuroinflammation in models of Alzheimer’s disease. Brain Plast 4(1):81–94
Calegari L et al (2018) Exercise training improves the IL-10/TNF-α cytokine balance in the gastrocnemius of rats with heart failure. Braz J Phys Ther 22(2):154–160
Cianciulli A et al (2015) IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int Immunopharmacol 24(2):369–376
Rosenzweig JM, Lei J, Burd I (2014) Interleukin-1 receptor blockade in perinatal brain injury. Front Pediatr 2:108
Scheller J et al (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813(5):878–888
Steensberg A et al (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285(2):E433–E437
Lu Y et al (2017) Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer’s disease. J Alzheim Dis 56(4):1469–1484
Saavedra A, Baltazar G, Duarte EP (2007) Interleukin-1beta mediates GDNF up-regulation upon dopaminergic injury in ventral midbrain cell cultures. Neurobiol Dis 25(1):92–104
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054):184–185
Cai H, Liang Q, Ge G (2016) Gypenoside attenuates β amyloid-induced inflammation in N9 microglial cells via SOCS1 signaling. Neural Plast 2016:6362707
Sajja VS, Hlavac N, VandeVord PJ (2016) Role of glia in memory deficits following traumatic brain injury: biomarkers of glia dysfunction. Front Integr Neurosci 10:7
Spielman LJ, Little JP, Klegeris A (2016) Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res Bull 125:19–29
López-Ortiz S et al (2021) Physical exercise and Alzheimer’s disease: effects on pathophysiological molecular pathways of the disease. Int J Mol Sci 22(6):2897
Li Z et al (2020) Physical exercise ameliorates the cognitive function and attenuates the neuroinflammation of Alzheimer’s disease via miR-129-5p. Dement Geriatr Cogn Disord 49(2):163–169
Heneka MT et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405
Abd El-Kader SM, Al-Jiffri OH (2016) Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer’s disease. Afr Health Sci 16(4):1045–1055
Wu C et al (2018) Beneficial effects of exercise pretreatment in a sporadic Alzheimer’s Rat model. Med Sci Sports Exerc 50(5):945–956
Pervaiz N, Hoffman-Goetz L (2012) Immune cell inflammatory cytokine responses differ between central and systemic compartments in response to acute exercise in mice. Exerc Immunol Rev 18:142–157
Hashiguchi D et al (2020) Resistance exercise decreases amyloid load and modulates inflammatory responses in the APP/PS1 mouse model for Alzheimer’s Disease. J Alzheim Dis 73(4):1525–1539
Liu Y et al (2020) The neuroprotective effect of Irisin in ischemic stroke. Front Aging Neurosci 12:588958
Rothaug M, Becker-Pauly C, Rose-John S (2016) The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta 1863(6 Pt A):1218–1227
Jensen CS et al (2019) Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp Gerontol 121:91–98
Colonna M (2003) TREMs in the immune system and beyond. Nat Rev Immunol 3(6):445–453
Forloni G, Balducci C (2018) Alzheimer’s disease, oligomers, and inflammation. J Alzheimers Dis 62(3):1261–1276
Choi SH et al (2018) Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361:6406
Balestrino R, Schapira AHV (2020) Parkinson disease. Eur J Neurol 27(1):27–42
Lotankar S, Prabhavalkar KS, Bhatt LK (2017) Biomarkers for Parkinson’s disease: recent advancement. Neurosci Bull 33(5):585–597
O’Callaghan A et al (2020) Comparing the influence of exercise intensity on brain-derived neurotrophic factor serum levels in people with Parkinson’s disease: a pilot study. Aging Clin Exp Res 32(9):1731–1738
Daniele SG et al (2015) Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci Signal 8(376):ra45
Dzamko N et al (2017) Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol 133(2):303–319
Béraud D et al (2013) Microglial activation and antioxidant responses induced by the Parkinson’s disease protein α-synuclein. J Neuroimmune Pharmacol 8(1):94–117
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18(2):101–113
Joers V et al (2017) Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog Neurobiol 155:57–75
Shabab T et al (2017) Neuroinflammation pathways: a general review. Int J Neurosci 127(7):624–633
Jang Y et al (2017) Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res 1655:186–193
Koo JH, Cho JY, Lee UB (2017) Treadmill exercise alleviates motor deficits and improves mitochondrial import machinery in an MPTP-induced mouse model of Parkinson’s disease. Exp Gerontol 89:20–29
Monteiro-Junior RS et al (2015) We need to move more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses 85(5):537–541
Tuon T et al (2012) Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 227:305–312
Dimatelis JJ et al (2013) Exercise partly reverses the effect of maternal separation on hippocampal proteins in 6-hydroxydopamine-lesioned rat brain. Exp Physiol 98(1):233–244
Eikelenboom P et al (2006) The significance of neuroinflammation in understanding Alzheimer’s disease. J Neural Transm (Vienna) 113(11):1685–1695
Singh SS et al (2020) NF-κB-mediated neuroinflammation in Parkinson’s Disease and potential therapeutic effect of polyphenols. Neurotox Res 37(3):491–507
Tuon T et al (2015) Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson’s Disease. Oxid Med Cell Longev 2015:261809
Orr CF, Rowe DB, Halliday GM (2002) An inflammatory review of Parkinson’s disease. Prog Neurobiol 68(5):325–340
Nikokalam Nazif N et al (2020) Effect of treadmill exercise on catalepsy and the expression of the BDNF gene in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson in male NMRI mice. Iran J Basic Med Sci 23(4):483–493
Shahidani S, Rajaei Z, Alaei H (2019) Pretreatment with crocin along with treadmill exercise ameliorates motor and memory deficits in hemiparkinsonian rats by anti-inflammatory and antioxidant mechanisms. Metab Brain Dis 34(2):459–468
Szymura J et al (2020) The immunomodulary effects of systematic exercise in older adults and people with Parkinson’s Disease. J Clin Med 9(1):184
Wu SY et al (2011) Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun 25(1):135–146
Zoladz JA et al (2014) Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J Physiol Pharmacol 65(3):441–448
Koo JH et al (2017) Treadmill exercise produces neuroprotective effects in a murine model of Parkinson’s disease by regulating the TLR2/MyD88/NF-κB signaling pathway. Neuroscience 356:102–113
Fickenscher H et al (2002) The interleukin-10 family of cytokines. Trends Immunol 23(2):89–96
Gonzalez-Aparicio R, Flores JA, Fernandez-Espejo E (2010) Antiparkinsonian trophic action of glial cell line-derived neurotrophic factor and transforming growth factor β1 is enhanced after co-infusion in rats. Exp Neurol 226(1):136–147
Zhou X, Spittau B, Krieglstein K (2012) TGFβ signalling plays an important role in IL4-induced alternative activation of microglia. J Neuroinflammation 9:210
Bent R et al (2018) Interleukin-1 beta-a friend or foe in malignancies? Int J Mol Sci 19(8):2155
Wang Y et al (2020) The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother 131:110660
Bronzuoli MR et al (2016) Targeting neuroinflammation in Alzheimer’s disease. J Inflamm Res 9:199–208
Neal M, Richardson JR (2018) Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim Biophys Acta Mol Basis Dis 1864(2):432–443
Wei H et al (2019) Interleukin-10 family cytokines immunobiology and structure. Adv Exp Med Biol 1172:79–96
Prado Lima MG et al (2018) Environmental enrichment and exercise are better than social enrichment to reduce memory deficits in amyloid beta neurotoxicity. Proc Natl Acad Sci USA 115(10):E2403-e2409
Katoh-Semba R et al (1997) Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem 69(1):34–42
von Bohlen Und Halbach O, von Bohlen Und Halbach V (2018) BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res 373(3):729–741
Lau YS et al (2011) Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur J Neurosci 33(7):1264–1274
Erickson KI et al (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108(7):3017–3022
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that it is an academic work and there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Almeida, E.J.R., Ibrahim, H.J., Chitolina Schetinger, M.R. et al. Modulation of Inflammatory Mediators and Microglial Activation Through Physical Exercise in Alzheimer’s and Parkinson's Diseases. Neurochem Res 47, 3221–3240 (2022). https://doi.org/10.1007/s11064-022-03713-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03713-x