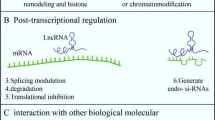
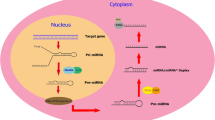
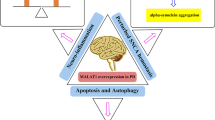
Abstract
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder and is associated with a range of motor and non-motor clinical symptoms. The underlying molecular pathogenesis of PD involves a variety of pathways and mechanisms, including α-synuclein proteostasis, mitochondrial dysfunction, oxidative stress, autophagy and apoptosis, neuroinflammation, and epigenetic regulation. Long non-coding RNAs (lncRNAs) are involved in the regulation of multiple pathological processes of PD. In this review, we provide an overview of large-scale studies on lncRNA expression profiling in PD patients and models, as well as highlight the impacts of lncRNAs on the pathogenesis of PD, which could provide basic information regarding the putative lncRNA-based biomarkers and therapeutic targets for the early diagnosis and treatment strategies for PD.

Similar content being viewed by others
References
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5(6):525–535
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222
Tang Y, Meng L, Wan CM, Liu ZH, Liao WH, Yan XX, Wang XY, Tang BS, Guo JF (2017) Identifying the presence of Parkinson’s disease using low-frequency fluctuations in BOLD signals. Neurosci Lett 645:1–6
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 3:17013
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318(1):121–134
Devos D, Moreau C, Dujardin K, Cabantchik I, Defebvre L, Bordet R (2013) New pharmacological options for treating advanced Parkinson’s disease. Clin Ther 35(10):1640–1652
Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta 1802(1):29–44
Lim SY, Lang AE (2010) The nonmotor symptoms of Parkinson’s disease--an overview. Mov Disord 25(Suppl 1):S123–S130
Wood LD, Neumiller JJ, Setter SM, Dobbins EK (2010) Clinical review of treatment options for select nonmotor symptoms of Parkinson’s disease. Am J Geriatr Pharmacother 8(4):294–315
Twelves D, Perkins KS, Counsell C (2003) Systematic review of incidence studies of Parkinson’s disease. Mov Disord 18(1):19–31
Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2013) Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol 70(7):859–866
Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29(13):1583–1590
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68(5):384–386
Nair VD, Ge Y (2016) Alterations of miRNAs reveal a dysregulated molecular regulatory network in Parkinson’s disease striatum. Neurosci Lett 629:99–104
Lyu Y, Bai L, Qin C (2019) Long noncoding RNAs in neurodevelopment and Parkinson’s disease. Animal Model Exp Med 2(4):239–251
Batista PJ, Chang HY (2013) Long noncoding RNAs: cellular address codes in development and disease. Cell 152(6):1298–1307
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12(12):861–874
Li L, Zhuang Y, Zhao X, Li X (2018) Long non-coding RNA in neuronal development and neurological disorders. Front Genet 9:744
Wei CW, Luo T, Zou SS, Wu AS (2018) The role of long noncoding RNAs in central nervous system and neurodegenerative diseases. Front Behav Neurosci 12:175
Quan Z, Zheng D, Qing H (2017) Regulatory roles of long non-coding RNAs in the central nervous system and associated neurodegenerative diseases. Front Cell Neurosci 11:175
Riva P, Ratti A, Venturin M (2016) The long non-coding RNAs in neurodegenerative diseases: novel mechanisms of pathogenesis. Curr Alzheimer Res 13(11):1219–1231
Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X et al (2012) The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22(9):1775–1789
Quinn JJ, Chang HY (2016) Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17(1):47–62
Dahariya S, Paddibhatla I, Kumar S, Raghuwanshi S, Pallepati A, Gutti RK (2019) Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol 112:82–92
Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annu Rev Biochem 81:145–166
Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136(4):629–641
Fatica A, Bozzoni I (2014) Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 15(1):7–21
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10(3):155–159
Soreq L, Guffanti A, Salomonis N, Simchovitz A, Israel Z, Bergman H, Soreq H (2014) Long non-coding RNA and alternative splicing modulations in Parkinson’s leukocytes identified by RNA sequencing. PLoS Comput Biol 10(3):e1003517
Zhou Y, Gu C, Li J, Zhu L, Huang G, Dai J, Huang H (2018) Aberrantly expressed long noncoding RNAs and genes in Parkinson’s disease. Neuropsychiatr Dis Treat 14:3219–3229
Chi LM, Wang LP, Jiao D (2019) Identification of differentially expressed genes and long noncoding RNAs associated with Parkinson’s disease. Parkinsons Dis 2019:6078251
Wang Q, Han CL, Wang KL, Sui YP, Li ZB, Chen N, Fan SY, Shimabukuro M, Wang F, Meng FG (2020) Integrated analysis of exosomal lncRNA and mRNA expression profiles reveals the involvement of lnc-MKRN2-42:1 in the pathogenesis of Parkinson’s disease. CNS Neurosci Ther 26(5):527–537
Fan Y, Li J, Yang Q, Gong C, Gao H, Mao Z, Yuan X, Zhu S, Xue Z (2019) Dysregulated long non-coding RNAs in Parkinson’s disease contribute to the apoptosis of human neuroblastoma cells. Front Neurosci 13:1320
Kraus TFJ, Haider M, Spanner J, Steinmaurer M, Dietinger V, Kretzschmar HA (2017) Altered long noncoding RNA expression precedes the course of Parkinson’s disease-a preliminary report. Mol Neurobiol 54(4):2869–2877
Ni Y, Huang H, Chen Y, Cao M, Zhou H, Zhang Y (2017) Investigation of long non-coding RNA expression profiles in the substantia Nigra of Parkinson’s disease. Cell Mol Neurobiol 37(2):329–338
Elkouris M, Kouroupi G, Vourvoukelis A, Papagiannakis N, Kaltezioti V, Matsas R, Stefanis L, Xilouri M, Politis PK (2019) Long non-coding RNAs associated with neurodegeneration-linked genes are reduced in Parkinson’s disease patients. Front Cell Neurosci 13:58
Wang L, Yang H, Wang Q, Zhang Q, Wang Z, Zhang Q, Wu S, Li H (2018) Paraquat and MPTP induce alteration in the expression profile of long noncoding RNAs in the substantia nigra of mice: role of the transcription factor Nrf2. Toxicol Lett 291:11–28
Li J, Sun Y, Chen J (2019) Transcriptome sequencing in a 6-hydroxydopamine rat model of Parkinson’s disease. Genes Genet Syst 94(2):61–69
Li XZ, Zhang SN, Lu F, Liu SM (2016) Microarray expression analysis for the paradoxical roles of Acanthopanax senticosus harms in treating alpha-Synucleinopathies. Phytother Res 30(2):243–252
Jiao F, Wang Q, Zhang P, Bu L, Yan J, Tian B (2017) Expression signatures of long non-coding RNA in the substantia nigra of pre-symptomatic mouse model of Parkinson’s disease. Behav Brain Res 331:123–130
Lin D, Liang Y, Jing X, Chen Y, Lei M, Zeng Z, Zhou T, Wu X, Peng S, Zheng D, Huang K, Yang L, Xiao S et al (1678) Microarray analysis of an synthetic alpha-synuclein induced cellular model reveals the expression profile of long non-coding RNA in Parkinson’s disease. Brain Res 2018:384–396
Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L (2011) Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol 10(11):1015–1025
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S et al (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46(9):989–993
Xilouri M, Brekk OR, Stefanis L (2013) Alpha-Synuclein and protein degradation systems: a reciprocal relationship. Mol Neurobiol 47(2):537–551
Carrieri C, Cimatti L, Biagioli M, Beugnet A, Zucchelli S, Fedele S, Pesce E, Ferrer I, Collavin L, Santoro C, Forrest AR, Carninci P, Biffo S et al (2012) Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 491(7424):454–457
Carrieri C, Forrest AR, Santoro C, Persichetti F, Carninci P, Zucchelli S, Gustincich S (2015) Expression analysis of the long non-coding RNA antisense to Uchl1 (AS Uchl1) during dopaminergic cells’ differentiation in vitro and in neurochemical models of Parkinson’s disease. Front Cell Neurosci 9:114
Zhang QS, Wang ZH, Zhang JL, Duan YL, Li GF, Zheng DL (2016) Beta-asarone protects against MPTP-induced Parkinson’s disease via regulating long non-coding RNA MALAT1 and inhibiting alpha-synuclein protein expression. Biomed Pharmacother 83:153–159
Xia D, Sui R, Zhang Z (2019) Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J Cell Biochem 120(4):4942–4951
Lin Q, Hou S, Dai Y, Jiang N, Lin Y (2019) LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson’s disease through RAB3IP. Biol Chem 400(9):1217–1228
Chen Y, Lian YJ, Ma YQ, Wu CJ, Zheng YK, Xie NC (2018) LncRNA SNHG1 promotes alpha-synuclein aggregation and toxicity by targeting miR-15b-5p to activate SIAH1 in human neuroblastoma SH-SY5Y cells. Neurotoxicology 68:212–221
Xu X, Zhuang C, Wu Z, Qiu H, Feng H, Wu J (2018) LincRNA-p21 inhibits cell viability and promotes cell apoptosis in Parkinson’s disease through activating alpha-Synuclein expression. Biomed Res Int 2018:8181374
Liu Y, Lu Z (2018) Long non-coding RNA NEAT1 mediates the toxic of Parkinson’s disease induced by MPTP/MPP+ via regulation of gene expression. Clin Exp Pharmacol Physiol 45(8):841–848
Lu M, Sun WL, Shen J, Wei M, Chen B, Qi YJ, Xu CS (2018) LncRNA-UCA1 promotes PD development by upregulating SNCA. Eur Rev Med Pharmacol Sci 22(22):7908–7915
Zhang LM, Wang MH, Yang HC, Tian T, Sun GF, Ji YF, Hu WT, Liu X, Wang JP, Lu H (2019) Dopaminergic neuron injury in Parkinson’s disease is mitigated by interfering lncRNA SNHG14 expression to regulate the miR-133b/ alpha-synuclein pathway. Aging (Albany NY) 11(21):9264–9279
Schapira AH (2007) Mitochondrial dysfunction in Parkinson’s disease. Cell Death Differ 14(7):1261–1266
Hu Q, Wang G (2016) Mitochondrial dysfunction in Parkinson’s disease. Transl Neurodegener 5:14
Bose A, Beal MF (2016) Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 139(Suppl 1):216–231
Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283(14):9089–9100
Bhattacharjee N, Borah A (2016) Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson’s disease. Neurochem Int 101:48–55
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3(4):461–491
Hwang O (2013) Role of oxidative stress in Parkinson’s disease. Exp Neurobiol 22(1):11–17
Scheele C, Petrovic N, Faghihi MA, Lassmann T, Fredriksson K, Rooyackers O, Wahlestedt C, Good L, Timmons JA (2007) The human PINK1 locus is regulated in vivo by a non-coding natural antisense RNA during modulation of mitochondrial function. BMC Genomics 8:74
Pickrell AM, Youle RJ (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85(2):257–273
Simchovitz A, Hanan M, Niederhoffer N, Madrer N, Yayon N, Bennett ER, Greenberg DS, Kadener S, Soreq H (2019) NEAT1 is overexpressed in Parkinson’s disease substantia nigra and confers drug-inducible neuroprotection from oxidative stress. FASEB J 33(10):11223–11234
Ding XM, Zhao LJ, Qiao HY, Wu SL, Wang XH (2019) Long non-coding RNA-p21 regulates MPP(+)-induced neuronal injury by targeting miR-625 and derepressing TRPM2 in SH-SY5Y cells. Chem Biol Interact 307:73–81
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49
Xiong N, Xiong J, Jia M, Liu L, Zhang X, Chen Z, Huang J, Zhang Z, Hou L, Luo Z, Ghoorah D, Lin Z, Wang T (2013) The role of autophagy in Parkinson’s disease: rotenone-based modeling. Behav Brain Funct 9:13
Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ (2012) The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med 2(4):a009357
Zhang L, Dong Y, Xu X, Xu Z (2012) The role of autophagy in Parkinson’s disease. Neural Regen Res 7(2):141–145
Sanchez-Perez AM, Claramonte-Clausell B, Sanchez-Andres JV, Herrero MT (2012) Parkinson’s disease and autophagy. Parkinsons Dis 2012:429524
Tatton WG, Chalmers-Redman R, Brown D, Tatton N (2003) Apoptosis in Parkinson’s disease: signals for neuronal degradation. Ann Neurol 53(Suppl 3):S61–S70 discussion S70-62
Liu W, Zhang Q, Zhang J, Pan W, Zhao J, Xu Y (2017) Long non-coding RNA MALAT1 contributes to cell apoptosis by sponging miR-124 in Parkinson disease. Cell Biosci 7:19
Chen Q, Huang X, Li R (2018) lncRNA MALAT1/miR-205-5p axis regulates MPP(+)-induced cell apoptosis in MN9D cells by directly targeting LRRK2. Am J Transl Res 10(2):563–572
Wang S, Zhang X, Guo Y, Rong H, Liu T (2017) The long noncoding RNA HOTAIR promotes Parkinson’s disease by upregulating LRRK2 expression. Oncotarget 8(15):24449–24456
Qian C, Ye Y, Mao H, Yao L, Sun X, Wang B, Zhang H, Xie L, Zhang H, Zhang Y, Zhang S, He X (2019) Downregulated lncRNA-SNHG1 enhances autophagy and prevents cell death through the miR-221/222 /p27/mTOR pathway in Parkinson’s disease. Exp Cell Res 384(1):111614
Yan W, Chen ZY, Chen JQ, Chen HM (2018) LncRNA NEAT1 promotes autophagy in MPTP-induced Parkinson’s disease through stabilizing PINK1 protein. Biochem Biophys Res Commun 496(4):1019–1024
Xie SP, Zhou F, Li J, Duan SJ (2019) NEAT1 regulates MPP(+)-induced neuronal injury by targeting miR-124 in neuroblastoma cells. Neurosci Lett 708:134340
Peng T, Liu X, Wang J, Liu Y, Fu Z, Ma X, Li J, Sun G, Ji Y, Lu J, Wan W, Lu H (2019) Long noncoding RNA HAGLROS regulates apoptosis and autophagy in Parkinson’s disease via regulating miR-100/ATG10 axis and PI3K/Akt/mTOR pathway activation. Artif Cells Nanomed Biotechnol 47(1):2764–2774
Song Q, Geng Y, Li Y, Wang L, Qin J (2019) Long noncoding RNA NORAD regulates MPP+-induced Parkinson’s disease model cells. J Chem Neuroanat 101:101668
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353(6301):777–783
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8(4):382–397
Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM (2008) Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci 28(30):7687–7698
Ye Y, He X, Lu F, Mao H, Zhu Z, Yao L, Luo W, Sun X, Wang B, Qian C, Zhang Y, Lu G, Zhang S (2018) A lincRNA-p21/miR-181 family feedback loop regulates microglial activation during systemic LPS- and MPTP- induced neuroinflammation. Cell Death Dis 9(8):803
Cao B, Wang T, Qu Q, Kang T, Yang Q (2018) Long noncoding RNA SNHG1 promotes Neuroinflammation in Parkinson’s disease via regulating miR-7/NLRP3 pathway. Neuroscience 388:118–127
Cai L, Tu L, Li T, Yang X, Ren Y, Gu R, Zhang Q, Yao H, Qu X, Wang Q, Tian J (2019) Downregulation of lncRNA UCA1 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson’s disease through the inhibition of the PI3K/Akt signaling pathway. Int Immunopharmacol 75:105734
Coupland KG, Mellick GD, Silburn PA, Mather K, Armstrong NJ, Sachdev PS, Brodaty H, Huang Y, Halliday GM, Hallupp M, Kim WS, Dobson-Stone C, Kwok JB (2014) DNA methylation of the MAPT gene in Parkinson’s disease cohorts and modulation by vitamin E in vitro. Mov Disord 29(13):1606–1614
Iwata A, Nagata K, Hatsuta H, Takuma H, Bundo M, Iwamoto K, Tamaoka A, Murayama S, Saido T, Tsuji S (2014) Altered CpG methylation in sporadic Alzheimer’s disease is associated with APP and MAPT dysregulation. Hum Mol Genet 23(3):648–656
Masliah E, Dumaop W, Galasko D, Desplats P (2013) Distinctive patterns of DNA methylation associated with Parkinson disease: identification of concordant epigenetic changes in brain and peripheral blood leukocytes. Epigenetics 8(10):1030–1038
Coupland KG, Kim WS, Halliday GM, Hallupp M, Dobson-Stone C, Kwok JB (2016) Role of the long non-coding RNA MAPT-AS1 in regulation of microtubule associated protein tau (MAPT) expression in Parkinson’s disease. PLoS One 11(6):e0157924
Funding
This work was supported by grants from the Liaoning Province Natural Science Foundation (20180550833) to CQX, and National stem cell clinical research registered project (CMR-20161129-1003) to JL.
Author information
Authors and Affiliations
Contributions
JL had the idea for the article, CQX performed the literature search, CQX and JL drafted and critically revised the work. Both authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Both authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xin, C., Liu, J. Long Non-coding RNAs in Parkinson’s Disease. Neurochem Res 46, 1031–1042 (2021). https://doi.org/10.1007/s11064-021-03230-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03230-3