Abstract
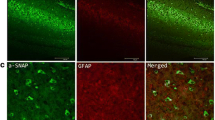
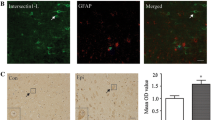
Peptidyl-prolyl cis–trans isomerase NIMA-interacting 1 (Pin1) is a unique PPIase belonging to the parvulin family, and it isomerizes peptide bond between phospho-(Ser/Thr) and Pro. Pin1 has been linked to the pathogenesis of various human diseases; however, its exact biological functions remain unclear. The aim of the present study is to explore the expression pattern of Pin1 in patients with refractory epilepsy and in a chronic pilocarpine-induced epileptic mouse model. Using Western blot, immunofluorescence and immunoprecipitation analysis, we found that Pin1 protein was mainly distributed in neurons, demonstrated by colocalization with the dendritic marker, MAP2. However, the expression of Pin1 decreased remarkably in epileptic patients and experimental mice. Furthermore, the reciprocal coimmunoprecipitation analysis showed that Pin1 interacted with NR2A and NR2B-containing NMDA receptors not AMPA receptors in epileptic mouse models. Our results are the first to indicate that the expression of Pin1 in epileptic brain tissue could play important roles in epilepsy.




Similar content being viewed by others
References
Rajendran S, Iyer A (2008) Epilepsy: addressing the transition from pediatric to adult care. Adolesc Health Med Ther 7:77–87
Duncan JS, Sander JW, Sisodiya SM, Walker MC (2006) Adult epilepsy. Lancet 367:1087–1100
Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat. Rev Neurol 7:31–40
Moshe SL, Perucca E, Ryvlin P, Tomson T (2015) Epilepsy: new advances. Lancet 385:884–898
Spencer S, Huh L (2008) Outcomes of epilepsy surgery in adults and children. Lancet Neurol 7:525–537
Javidan M (2012) Electroencephalography in mesial temporal lobe epilepsy: a review. Epilepsy Res Treat 2012:637430
Cendes F, Kobayashi E, Lopes-Cendes I (2005) Familial temporal lobe epilepsy with auditory features. Epilepsia 10:59–60
Henshall DC, Engel T (2013) Contribution of apoptosis-associated signaling pathways to epileptogenesis: lessons from Bcl-2 family knockouts. Front Cell Neurosci 7:110
Lu KP, Zhou XZ (2007) The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol 8:904–916
Lee TH, Pastorino L, Lu KP (2011) Peptidyl-prolyl cis–trans isomerase Pin1 in ageing, cancer and Alzheimer disease. Expert Rev Mol Med 13:e21
Girardini JE, Napoli M, Piazza S et al (2011) A Pin1/mutant p53 axis promotes aggressiveness in breast cancer. Cancer Cell 20:79–91
Liou YC, Zhou XZ, Lu KP (2011) Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci 36:501–514
Yuan WC, Lee YR, Huang SF et al (2011) A Cullin3-KLHL20 Ubiquitin ligase-dependent pathway targets PML to potentiate HIF-1 signaling and prostate cancer progression. Cancer Cell 20:214–228
Driver JA, Lu KP (2010) Pin1: a new genetic link between Alzheimer’s disease, cancer and aging. Curr Aging Sci 3:158–165
Rudrabhatla P, Pant HC (2010) Phosphorylation-specific peptidyl-prolyl isomerization of neuronal cytoskeletal proteins by Pin1: implications for therapeutics in neurodegeneration. J Alzheimers Dis 19:389–403
Zita MM, Marchionni I, Bottos E et al (2007) Zacchi, Post-phosphorylation prolyl isomerisation of gephyrin represents a mechanism to modulate glycine receptors function. EMBO J 26:1761–1771
Fang M, Shen L et al (2011) Downregulation of gephyrin in temporal lobe epilepsy neurons in humans and a rat model. Synapse 65:1006–1014
Zhu BL, Zha JS, Long Y et al (2016) Increased expression of copine VI in patients with refractory epilepsy and a rat model. J Neurol Sci 360:30–36
Lai YJ, Hu XT, Chen GJ, Wang XF, Zhu BL (2016) Down-regulation of adenylate kinase 5 in temporal lobe epilepsy patients and rat model. J Neurol Sci 366:20–26
Kwan P, Arzimanoglou A, Berg AT et al (2010) French, Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia 51:1069–1077
Vignoli T, Nehlig A, Massironi SG et al (2012) Consequences of pilocarpine-induced status epilepticus in immunodeficient mice. Brain Res 1450:125–137
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Antonelli R, De Filippo R, Middei S et al (2016) Pin1 modulates the synaptic content of NMDA receptors via prolyl-isomerization of PSD-95. J Neurosci 36:5437–5447
Butterfield DA, Poon HF, St Clair D et al (2006) Markesbery, redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis 22:223–232
Sultana R, Boyd-Kimball D, Poon HF et al (2006) Oxidative modification and downregulation of Pin1 in Alzheimer’s disease hippocampus: a redox proteomics analysis. Neurobiol Aging 27:918–925
Keeney JTR, Swomley AM, Harris JL et al (2012) Cell cycle proteins in brain in mild cognitive impairment: insights into progression to Alzheimer disease. Neurotox Res 22:220–230
Van Liefferinge J, Massie A, Portelli J et al (2013) Are vesicular neurotransmitter transporters potential treatment targets for temporal lobe epilepsy. Front Cell Neurosci 7:139
Ladépêche L, Dupuis JP, Groc L (2014) Surface trafficking of NMDA receptors: gathering from a partner to another. Semin Cell Dev Biol 27:3–13
Naylor DE, Liu H, Niquet J, Wasterlain CG (2013) Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol Dis 54:225–238
Frasca A, Aalbers M, Friqerio F et al (2011) Misplaced NMDA receptors in epileptogenesis contribute to excitotoxicity. Neurobiol Dis 43:507–515
Seo DW, Lopez-Merza ML, Allen S et al (2009) Contribution of a mitochondrial pathway to excitotoxic neuronal necrosis. J Neurosci Res 87:2087–2094
Lee K, Goodman L, Fourie C, Schenk S, Leitch B, Montgomery JM (2016) AMPA receptors as therapeutic targets for neurological disorders. Adv Protein Chem Struct Biol 103:203–261
Groc L, Bard L, Choquet D (2009) Surface trafficking of N-methyl-D-aspartate receptors: physiological and pathological perspectives. Neuroscience 158:4–18
Lau CG, Zukin RS (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426
Cai ZX, Li S, Li S (2016) Antagonist targeting microRNA-155 protects against lithium-pilocarpine-induced status epilepticus in C57BL/6 mice by activating brain-derived neurotrophic factor. Front Pharmacol 7:129
Otsuka S, Ohkido T, Itakura M et al (2016) Dual mechanisms of rapid expression of anxiety-related behavior in pilocarpine-treated epileptic mice. Epilepsy Res 123:55–67
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 31500821) and the National Clinical Key Specialty Construction Foundation of China. We are grateful for the support of The Affiliated Children’s Hospital of Capital Institute of Pediatrics, The First Affiliated Hospital of PLA General Hospital and The First Affiliated Hospital of Chongqing Medical University, which supplied patient brain tissues. We also thank all patients and their families for their participation in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest
Rights and permissions
About this article
Cite this article
Tang, L., Zhang, Y., Chen, G. et al. Down-regulation of Pin1 in Temporal Lobe Epilepsy Patients and Mouse Model. Neurochem Res 42, 1211–1218 (2017). https://doi.org/10.1007/s11064-016-2158-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2158-8