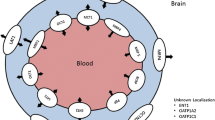
Abstract
Blood–brain barrier formed by brain capillary endothelial cells, being in contact with astrocytes endfeet and pericytes, separates extracellular fluid from plasma. Supply of necessary nutrients and removal of certain metabolites takes place due to the activity of transporting proteins from ABC (ATP binding cassette) and SLC (solute carrier) superfamilies. This review is focused on the SLC families involved in transport though the blood–brain barrier of energetic substrates (glucose, monocarboxylates, creatine), amino acids, neurotransmitters and their precursors, as well as organic ions. Members of SLC1, SLC2, SLC3/SLC7, SLC5, SLC6, SLC16, SLC22, SLC38, SLC44, SLC47 and SLCO (SLC21), whose presence in the blood–brain barriers has been demonstrated are characterized with a special emphasis put on polarity of transporters localization in a luminal (blood side) versus an abluminal (brain side) membrane.




Similar content being viewed by others
Notes
Please, note that original nomenclature SLCnXm (for Solute carrier), where n—number of family, X—subfamily, m—individual family member written in italics, was applied for human genome project and now is also used for transporting proteins (SLCnXm).
References
Hartsock A, Nelson WJ (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–669
Abbott NJ (1001) Permeability and transport of glial blood–brain barriers. Ann N Y Acad Sci 633:378–394
Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC (2013) The blood–brain barrier: an engineering perspective. Front Neuroeng 6:7. doi:10.3389/fnegg.2013.00007
Lewandowsky M (1890) Zur Lehre von der Cerebrospinalflussigkeit. Z Klin Med 40:480–494
Enerson BE, Drewes LR (2006) The rat blood–brain barrier transcriptome. J Cereb Blood Flow Metab 26:959–973
Dahlin A, Royall J, Hohmann JG, Wang J (2009) Expression profiling of the solute carrier gene family in the mouse brain. J Pharmacol Exp Ther 329:558–570
Geier EG, Chen EC, Webb A, Papp AC, Yee SW, Sadee W, Giacomini KM (2013) Profiling solute carrier transporters in the human blood–brain barrier. Clin Pharmacol Ther 94:636–639
Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T (2011) Quantitative targeted absolute proteomics of human blood–brain barrier transporters and receptors. J Neurochem 117:333–345
Uchida Y, Tachikawa M, Obuchi W, Hoshi Y, Tomioka Y, Ohtsuki S, Terasaki T (2013) A study protocol for quantitative targeted absolute proteomics (QTAP) by LC-MS/MS: application for inter-strain differences in protein expression levels of transporters, receptors, claudin-5, and marker proteins at the blood–brain barrier in ddY, FVB, and C57BL/6 J mice. Fluids Barriers CNS 10:21
Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK (2008) Subcellular localization of transporters along the rat blood–brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 155:423–438
Cornford EM, Hyman S (2005) Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx 2:27–43
Lee WJ, Hawkins RA, Vina JR, Peterson DR (1998) Glutamine transport by the blood–brain barrier: a possible mechanism for nitrogen removal. Am J Physiol 274:C1101–C1107
O’Kane RL, Hawkins RA (2003) Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood–brain barrier. Am J Physiol Endocrinol Metab 285:E1167–E1173
O’Kane RL, Vina JR, Simpson I, Zaragoza R, Mokashi A, Hawkins RA (2006) Cationic amino acid transport across the blood–brain barrier is mediated exclusively by system y+. Am J Physiol Endocrinol Metab 291:E412–E419
Qosa H, Miller DS, Pasinelli P, Trotti D (2015) Regulation of ABC efflux transporters at blood–brain barrier in health and neurological disorders. Brain Res 1628:298–316
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791
Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J (1987) Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 18:551–561
Ebert D, Haller RG, Walton ME (2003) Energy contribution of octanoate to intact rat brain metabolism measured by 13 C nuclear magnetic resonance spectroscopy. J Neurosci 23:5928–5935
Nehlig A, Pereira de Vasconcelos A (1993) Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol 40:163–221
Brown AM (2004) Brain glycogen re-awakened. J Neurochem 89:537–552
Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ (1998) Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 20:291–299
Pellerin L, Magistretti PJ (2004) Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 10:53–62
Dick AP, Harik SI, Klip A, Walker DM (1984) Identification and characterization of the glucose transporter of the blood–brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci USA 81:7233–7237
Pardridge WM, Boado RJ, Farrell CR (1990) Brain-type glucose transporter (GLUT-1) is selectively localized to the blood–brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem 265:18035–18040
Boado RJ, Pardridge WM (1994) Measurement of blood–brain barrier GLUT1 glucose transporter and actin mRNA by a quantitative polymerase chain reaction assay. J Neurochem 62:2085–2090
Farrell CL, Pardridge WM (1991) Blood–brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci USA 88:5779–5783
Simpson IA, Vannucci SJ, DeJoseph MR, Hawkins RA (2001) Glucose transporter asymmetries in the bovine blood–brain barrier. J Biol Chem 276:12725–12729
Cornford EM, Hyman S, Pardridge WM (1993) An electron microscopic immunogold analysis of developmental up-regulation of the blood–brain barrier GLUT1 glucose transporter. J Cereb Blood Flow Metab 13:841–854
Gerhart DZ, LeVasseur RJ, Broderius MA, Drewes LR (1989) Glucose transporter localization in brain using light and electron immunocytochemistry. J Neurosci Res 22:464–472
Witters LA, Vater CA, Lienhard GE (1985) Phosphorylation of the glucose transporter in vitro and in vivo by protein kinase C. Nature 315:777–778
Devraj K, Klinger ME, Myers RL, Mokashi A, Hawkins RA, Simpson IA (2011) GLUT-1 glucose transporters in the blood–brain barrier: differential phosphorylation. J Neurosci Res 89:1913–1925
Pouliot JF, Beliveau R (1995) Palmitoylation of the glucose transporter in blood–brain barrier capillaries. Biochim Biophys Acta 1234:191–196
Maher F, Vannucci SJ, Simpson IA (1993) Glucose transporter isoforms in brain: absence of GLUT3 from the blood–brain barrier. J Cereb Blood Flow Metab 13:342–345
Morgello S, Uson RR, Schwartz EJ, Haber RS (1995) The human blood–brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia 14:43–54
Regina A, Morchoisne S, Borson ND, McCall AL, Drewes LR, Roux F (2001) Factor(s) released by glucose-deprived astrocytes enhance glucose transporter expression and activity in rat brain endothelial cells. Biochim Biophys Acta 1540:233–242
Meireles M, Martel F, Araujo J, Santos-Buelga C, Gonzalez-Manzano S, Duenas M, de Freitas V, Mateus N, Calhau C, Faria A (2013) Characterization and modulation of glucose uptake in a human blood–brain barrier model. J Membr Biol 246:669–677
De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI (1991) Defective glucose transport across the blood–brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med 325:703–709
Gordon N, Newton RW (2003) Glucose transporter type1 (GLUT-1) deficiency. Brain Dev 25:477–480
Haberlandt E, Karall D, Jud V, Baumgartner SS, Zotter S, Rostasy K, Baumann M, Scholl-Buergi S (2014) Glucose transporter type 1 deficiency syndrome effectively treated with modified Atkins diet. Neuropediatrics 45:117–119
Rose RC (1988) Transport of ascorbic acid and other water-soluble vitamins. Biochim Biophys Acta 947:335–366
Burzle M, Hediger MA (2012) Functional and physiological role of vitamin C transporters. Curr Top Membr 70:357–375
Nowis D, Malenda A, Furs K, Oleszczak B, Sadowski R, Chlebowska J, Firczuk M, Bujnicki JM, Staruch AD, Zagozdzon R, Glodkowska-Mrowka E, Szablewski L, Golab J (2014) Statins impair glucose uptake in human cells. BMJ Open Diabetes Res Care 2:e000017
Elfeber K, Kohler A, Lutzenburg M, Osswald C, Galla HJ, Witte OW, Koepsell H (2004) Localization of the Na+-d-glucose cotransporter SGLT1 in the blood–brain barrier. Histochem Cell Biol 121:201–207
Vemula S, Roder KE, Yang T, Bhat GJ, Thekkumkara TJ, Abbruscato TJ (2009) A functional role for sodium-dependent glucose transport across the blood–brain barrier during oxygen glucose deprivation. J Pharmacol Exp Ther 328:487–495
Erokhova L, Horner A, Ollinger N, Siligan C, Pohl P (2016) The sodium glucose cotransporter SGLT1 is an extremely efficient facilitator of passive water transport. J Biol Chem 291:9712–9720
Halestrap AP, Meredith D (2004) The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447:619–628
Halestrap AP, Wilson MC (2012) The monocarboxylate transporter family–role and regulation. IUBMB Life 64:109–119
Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS (1994) Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76:865–873
Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP (1998) Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport l-lactate as well as butyrate. J Physiol 513:719–732
Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR (1997) Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol 273:E207–E213
Leino RL, Gerhart DZ, Drewes LR (1999) Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res Dev Brain Res 113:47–54
Broer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ (1997) Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem 272:30096–30102
Broer S, Schneider HP, Broer A, Rahman B, Hamprecht B, Deitmer JW (1998) Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J 333:167–174
Uhernik AL, Tucker C, Smith JP (201) Control of MCT1 function in cerebrovascular endothelial cells by intracellular pH. Brain Res 1376:10–22
Moschen I, Broer A, Galic S, Lang F, Broer S (2012) Significance of short chain fatty acid transport by members of the monocarboxylate transporter family (MCT). Neurochem Res 37:2562–2568
Tsuji A, Saheki A, Tamai I, Terasaki T (1993) Transport mechanism of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors at the blood–brain barrier. J Pharmacol Exp Ther 267:1085–1090
Vijay N, Morris ME (2014) Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des 20:1487–1498
Pardridge WM, Oldendorf WH (1977) Transport of metabolic substrates through the blood–brain barrier. J Neurochem 28:5–12
Smith JP, Drewes LR (2006) Modulation of monocarboxylic acid transporter-1 kinetic function by the cAMP signaling pathway in rat brain endothelial cells. J Biol Chem 281:2053–2060
Liu Z, Sneve M, Haroldson TA, Smith JP, Drewes LR (2016) Regulation of monocarboxylic acid transporter 1 trafficking by the canonical Wnt/beta-catenin pathway in rat brain endothelial cells requires cross-talk with notch signaling. J Biol Chem 291:8059–8069
Soares RV, Do TM, Mabondzo A, Pons G, Chhun S (2016) Ontogeny of ABC and SLC transporters in the microvessels of developing rat brain. Fundam Clin Pharmacol 30:107–116
Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP(2000) CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 19:3896–3904
Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR (2001) Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int 38:519–527
Ito K, Uchida Y, Ohtsuki S, Aizawa S, Kawakami H, Katsukura Y, Kamiie J, Terasaki T (2011) Quantitative membrane protein expression at the blood–brain barrier of adult and younger cynomolgus monkeys. J Pharm Sci 100:3939–3950
Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ (2003) Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135
Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C (2015) Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. doi:10.1016/j.neuroscience.2015.09.070
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281:21–40
Ohtsuki S, Tachikawa M, Takanaga H, Shimizu H, Watanabe M, Hosoya K, Terasaki T (2002) The blood–brain barrier creatine transporter is a major pathway for supplying creatine to the brain. J Cereb Blood Flow Metab 22:1327–1335
Broer S, Gether U (2012) The solute carrier 6 family of transporters. Br J Pharmacol 167:256–278
Beard E, Braissant O (2010) Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem 115:297–313
Salomons GS, van Dooren SJ, Verhoeven NM, Marsden D, Schwartz C, Cecil KM, DeGrauw TJ, Jakobs C (2003) X-linked creatine transporter defect: an overview. J Inherit Metab Dis 26:309–318
Mancardi MM, Caruso U, Schiaffino MC, Baglietto MG, Rossi A, Battaglia FM, Salomons GS, Jakobs C, Zara F, Veneselli E, Gaggero R (2007) Severe epilepsy in X-linked creatine transporter defect (CRTR-D). Epilepsia 48:1211–1213
Thurm A, Himelstein D, D’Souza P, Rennert O, Jiang S, Olatunji D, Longo N, Pasquali M, Swedo S, Salomons GS, Carrillo N (2016) Creatine transporter deficiency: screening of males with neurodevelopmental disorders and neurocognitive characterization of a case. J Dev Behav Pediatr 37:322–326
Braissant O, Cagnon L, Monnet-Tschudi F, Speer O, Wallimann T, Honegger P, Henry H (2008) Ammonium alters creatine transport and synthesis in a 3D culture of developing brain cells, resulting in secondary cerebral creatine deficiency. Eur J Neurosci 27:1673–1685
Braissant O (2010) Ammonia toxicity to the brain: effects on creatine metabolism and transport and protective roles of creatine. Mol Genet Metab 100(Suppl 1):S53–S58
Braissant O (2012) Creatine and guanidinoacetate transport at blood–brain and blood-cerebrospinal fluid barriers. J Inherit Metab Dis 35:655–664
Westergren I, Nystrom B, Hamberger A, Nordborg C, Johansson BB (1994) Concentrations of amino acids in extracellular fluid after opening of the blood–brain barrier by intracarotid infusion of protamine sulfate. J Neurochem 62:159–165
Oldendorf WH (1971) Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol 221:1629–1639
Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM (1999) Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc Natl Acad Sci USA 96:12079–12084
Fotiadis D, Kanai Y, Palacin M (2013) The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34:139–158
Kageyama T, Nakamura M, Matsuo A, Yamasaki Y, Takakura Y, Hashida M, Kanai Y, Naito M, Tsuruo T, Minato N, Shimohama S (2000) The 4F2hc/LAT1 complex transports l-DOPA across the blood–brain barrier. Brain Res 879:115–121
Dickens D, Webb SD, Antonyuk S, Giannoudis A, Owen A, Radisch S, Hasnain SS, Pirmohamed M (2013) Transport of gabapentin by LAT1 (SLC7A5). Biochem Pharmacol 85:1672–1683
Duelli R, Enerson BE, Gerhart DZ, Drewes LR (2000) Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab 20:1557–1562
Boado RJ, Li JY, Tsukamoto H, Pardridge WM (2003) Hypoxia induces de-stabilization of the LAT1 large neutral amino acid transporter mRNA in brain capillary endothelial cells. J Neurochem 85:1037–1042
Sloan JL, Mager S (1999) Cloning and functional expression of a human Na(+) and Cl(−)-dependent neutral and cationic amino acid transporter B(0+). J Biol Chem 274:23740–23745
Berezowski V, Miecz D, Marszalek M, Broer A, Broer S, Cecchelli R, Nalecz KA (2004) Involvement of OCTN2 and B0,+ in the transport of carnitine through an in vitro model of the blood–brain barrier. J Neurochem 91:860–872
Czeredys M, Mysiorek C, Kulikova N, Samluk L, Berezowski V, Cecchelli R, Nalecz K (2008) A polarized localization of amino acid/carnitine transporter B0,+ (ATB0,+) in the blood–brain barrier. Biochem Biophys Res Commun 376:267–270
Michalec K, Mysiorek C, Kuntz M, Berezowski V, Szczepankiewicz AA, Wilczynski GM, Cecchelli R, Nalecz KA (2014) Protein kinase C restricts transport of carnitine by amino acid transporter ATB(0,+) apically localized in the blood–brain barrier. Arch Biochem Biophys 554:28–35
Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, McKenna MC (2010) Metabolism of acetyl-l-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem 114:820–831
Nakanishi T, Hatanaka T, Huang W, Prasad PD, Leibach FH, Ganapathy ME, Ganapathy V (2001) Na+- and Cl−-coupled active transport of carnitine by the amino acid transporter ATB(0,+) from mouse colon expressed in HRPE cells and Xenopus oocytes. J Physiol 532:297–304
Ganapathy ME, Huang W, Rajan DP, Carter AL, Sugawara M, Iseki K, Leibach FH, Ganapathy V (2000) Beta-lactam antibiotics as substrates for OCTN2, an organic cation/carnitine transporter. J Biol Chem 275:1699–1707
Samluk L, Czeredys M, Skowronek K, Nalecz KA (2012) Protein kinase C regulates amino acid transporter ATB(0+). Biochem Biophys Res Commun 422:64–69
Hatanaka T, Huang W, Nakanishi T, Bridges CC, Smith SB, Prasad PD, Ganapathy ME, Ganapathy V (2002) Transport of d-serine via the amino acid transporter ATB(0,+) expressed in the colon. Biochem Biophys Res Commun 291:291–295
Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K (1995) Functional comparison of d-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem 65:454–458
Wolosker H, Blackshaw S, Snyder SH (1999) Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA 96:13409–13414
Ennis SR, Kawai N, Ren XD, Abdelkarim GE, Keep RF (1998) Glutamine uptake at the blood–brain barrier is mediated by N-system transport. J Neurochem 71:2565–2573
Xiang J, Ennis SR, Abdelkarim GE, Fujisawa M, Kawai N, Keep RF (2003) Glutamine transport at the blood–brain and blood-cerebrospinal fluid barriers. Neurochem Int 43:279–288
Ruderisch N, Virgintino D, Makrides V, Verrey F (2011) Differential axial localization along the mouse brain vascular tree of luminal sodium-dependent glutamine transporters Snat1 and Snat3. J Cereb Blood Flow Metab 31:1637–1647
Boulland JL, Rafiki A, Levy LM, Storm-Mathisen J, Chaudhry FA (2003) Highly differential expression of SN1, a bidirectional glutamine transporter, in astroglia and endothelium in the developing rat brain. Glia 41:260–275
Dolgodilina E, Imobersteg S, Laczko E, Welt T, Verrey F, Makrides V (2015) Brain interstitial fluid glutamine homeostasis is controlled by blood–brain barrier SLC7A5/LAT1 amino acid transporter. J Cereb Blood Flow Metab. doi:10.1177/0271678X15609331
Stoll J, Wadhwani KC, Smith QR (1993) Identification of the cationic amino acid transporter (System y+) of the rat blood–brain barrier. J Neurochem 60:1956–1959
Skowronska M, Zielinska M, Wojcik-Stanaszek L, Ruszkiewicz J, Milatovic D, Aschner M, Albrecht J (2012) Ammonia increases paracellular permeability of rat brain endothelial cells by a mechanism encompassing oxidative/nitrosative stress and activation of matrix metalloproteinases. J Neurochem 121:125–134
Zielinska M, Popek M, Albrecht J (2014) Roles of changes in active glutamine transport in brain edema development during hepatic encephalopathy: an emerging concept. Neurochem Res 39:599–604
Hosoya K, Sugawara M, Asaba H, Terasaki T (1999) Blood–brain barrier produces significant efflux of L-aspartic acid but not d-aspartic acid: in vivo evidence using the brain efflux index method. J Neurochem 73:1206–1211
Jensen AA, Fahlke C, Bjorn-Yoshimoto WE, Bunch L (2015) Excitatory amino acid transporters: recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Curr Opin Pharmacol 20:116–123
O’Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA (1999) Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood–brain barrier. A mechanism for glutamate removal. J Biol Chem 274:31891–31895
Cederberg HH, Uhd NC, Brodin B (2014) Glutamate efflux at the blood–brain barrier: cellular mechanisms and potential clinical relevance. Arch Med Res 45:639–645
Helms HC, Madelung R, Waagepetersen HS, Nielsen CU, Brodin B (2012) In vitro evidence for the brain glutamate efflux hypothesis: brain endothelial cells cocultured with astrocytes display a polarized brain-to-blood transport of glutamate. Glia 60:882–893
Hosoya K, Tomi M, Ohtsuki S, Takanaga H, Saeki S, Kanai Y, Endou H, Naito M, Tsuruo T, Terasaki T (2002) Enhancement of L-cystine transport activity and its relation to xCT gene induction at the blood–brain barrier by diethyl maleate treatment. J Pharmacol Exp Ther 302:225–231
Brigham MP, Stein WH, Moore S (1960) The concentrations of cysteine and cystine in human blood plasma. J Clin Invest 39:1633–1638
Karelson E, Bogdanovic N, Garlind A, Winblad B, Zilmer K, Kullisaar T, Vihalemm T, Kairane C, Zilmer M (2001) The cerebrocortical areas in normal brain aging and in Alzheimer’s disease: noticeable differences in the lipid peroxidation level and in antioxidant defense. Neurochem Res 26:353–361
Tetsuka K, Takanaga H, Ohtsuki S, Hosoya K, Terasaki T (2003) The l-isomer-selective transport of aspartic acid is mediated by ASCT2 at the blood–brain barrier. J Neurochem 87:891–901
Tayarani I, Lefauconnier JM, Roux F, Bourre JM (1987) Evidence for an alanine, serine, and cysteine system of transport in isolated brain capillaries. J Cereb Blood Flow Metab 7:585–591
Sakai K, Shimizu H, Koike T, Furuya S, Watanabe M (2003) Neutral amino acid transporter ASCT1 is preferentially expressed in l-ser-synthetic/storing glial cells in the mouse brain with transient expression in developing capillaries. J Neurosci 23:550–560
Sanchez del Pino MM, Hawkins RA, Peterson DR (1992) Neutral amino acid transport by the blood–brain barrier. Membrane vesicle studies. J Biol Chem 267:25951–25957
Takanaga H, Tokuda N, Ohtsuki S, Hosoya K, Terasaki T (2002) ATA2 is predominantly expressed as system A at the blood–brain barrier and acts as brain-to-blood efflux transport for l-proline. Mol Pharmacol 61:1289–1296
Alfieri RR, Petronini PG, Bonelli MA, Caccamo AE, Cavazzoni A, Borghetti AF, Wheeler KP (2001) Osmotic regulation of ATA2 mRNA expression and amino acid transport System A activity. Biochem Biophys Res Commun 283:174–178
O’Kane RL, Vina JR, Simpson I, Hawkins RA (2004) Na+ -dependent neutral amino acid transporters A, ASC, and N of the blood–brain barrier: mechanisms for neutral amino acid removal. Am J Physiol Endocrinol Metab 287:E622–E629
Sanchez del Pino MM, Peterson DR, Hawkins RA (1995) Neutral amino acid transport characterization of isolated luminal and abluminal membranes of the blood–brain barrier. J Biol Chem 270:14913–14918
Takanaga H, Mackenzie B, Peng JB, Hediger MA (2005) Characterization of a branched-chain amino-acid transporter SBAT1 (SLC6A15) that is expressed in human brain. Biochem Biophys Res Commun 337:892–900
Anderson CM, Ganapathy V, Thwaites DT (2008) Human solute carrier SLC6A14 is the beta-alanine carrier. J Physiol 586:4061–4067
Kang YS, Ohtsuki S, Takanaga H, Tomi M, Hosoya K, Terasaki T (2002) Regulation of taurine transport at the blood–brain barrier by tumor necrosis factor-alpha, taurine and hypertonicity. J Neurochem 83:1188–1195
Albrecht J, Schousboe A (2005) Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res 30:1615–1621
Hilgier W, Oja SS, Saransaari P, Albrecht J (2005) Taurine prevents ammonia-induced accumulation of cyclic GMP in rat striatum by interaction with GABAA and glycine receptors. Brain Res 1043:242–246
Hilgier W, Olson JE, Albrecht J (1996) Relation of taurine transport and brain edema in rats with simple hyperammonemia or liver failure. J Neurosci Res 45:69–74
Faff L, Reichenbach A, Albrecht J (1997) Two modes of stimulation by ammonia of taurine release from cultured rabbit Muller cells. Neurochem Int 31:301–305
Zielinska M, Zablocka B, Albrecht J (2003) Effect of ammonia on taurine transport in C6 glioma cells. Adv Exp Med Biol 526:463–470
Lee NY, Kang YS (2004) The brain-to-blood efflux transport of taurine and changes in the blood–brain barrier transport system by tumor necrosis factor-alpha. Brain Res 1023:141–147
Rasgado-Flores H, Mokashi A, Hawkins RA (2012) Na(+)-dependent transport of taurine is found only on the abluminal membrane of the blood–brain barrier. Exp Neurol 233:457–462
Broer S, Brookes N (2001) Transfer of glutamine between astrocytes and neurons. J Neurochem 77:705–719
Skowronska M, Albrecht J (2012) Alterations of blood brain barrier function in hyperammonemia: an overview. Neurotox Res 21:236–244
Kakee A, Takanaga H, Terasaki T, Naito M, Tsuruo T, Sugiyama Y (2001) Efflux of a suppressive neurotransmitter, GABA, across the blood–brain barrier. J Neurochem 79:110–118
Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N (1993) Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain. J Biol Chem 268:2106–2112
Takanaga H, Ohtsuki S, Hosoya K, Terasaki T (2001) GAT2/BGT-1 as a system responsible for the transport of gamma-aminobutyric acid at the mouse blood–brain barrier. J Cereb Blood Flow Metab 21:1232–1239
Al-Sarraf H (2002) Transport of 14 C-gamma-aminobutyric acid into brain, cerebrospinal fluid and choroid plexus in neonatal and adult rats. Brain Res Dev Brain Res 139:121–129
Wakayama K, Ohtsuki S, Takanaga H, Hosoya K, Terasaki T (2002) Localization of norepinephrine and serotonin transporter in mouse brain capillary endothelial cells. Neurosci Res 44:173–180
Young LW, Darios ES, Watts SW (2015) An immunohistochemical analysis of SERT in the blood–brain barrier of the male rat brain. Histochem Cell Biol 144:321–329
Allen DD, Smith QR (2001) Characterization of the blood–brain barrier choline transporter using the in situ rat brain perfusion technique. J Neurochem 76:1032–1041
Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I (2000) Identification and characterization of the high-affinity choline transporter. Nat Neurosci 3:120–125
Friedrich A, George RL, Bridges CC, Prasad PD, Ganapathy V (2001) Transport of choline and its relationship to the expression of the organic cation transporters in a rat brain microvessel endothelial cell line (RBE4). Biochim Biophys Acta 1512:299–307
Iwao B, Yara M, Hara N, Kawai Y, Yamanaka T, Nishihara H, Inoue T, Inazu M (2016) Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochem Int 93:40–50
Pacholczyk T, Blakely RD, Amara SG (1991) Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 350:350–354
Lincoln J (1995) Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther 68:473–501
Lasbennes F, Sercombe R, Seylaz J (1983) Monoamine oxidase activity in brain microvessels determined using natural and artificial substrates: relevance to the blood–brain barrier. J Cereb Blood Flow Metab 3:521–528
Hosoya K, Tachikawa M (2011) Roles of organic anion/cation transporters at the blood–brain and blood-cerebrospinal fluid barriers involving uremic toxins. Clin Exp Nephrol 15:478–485
Ohtsuki S, Asaba H, Takanaga H, Deguchi T, Hosoya K, Otagiri M, Terasaki T (2002) Role of blood–brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem 83:57–66
Mori S, Takanaga H, Ohtsuki S, Deguchi T, Kang YS, Hosoya K, Terasaki T (2003) Rat organic anion transporter 3 (rOAT3) is responsible for brain-to-blood efflux of homovanillic acid at the abluminal membrane of brain capillary endothelial cells. J Cereb Blood Flow Metab 23:432–440
Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB (2003) Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol Renal Physiol 284:F763–F769
Kikuchi R, Kusuhara H, Sugiyama D, Sugiyama Y (2003) Contribution of organic anion transporter 3 (Slc22a8) to the elimination of p-aminohippuric acid and benzylpenicillin across the blood–brain barrier. J Pharmacol Exp Ther 306:51–58
Kikuchi T, Okamura T, Wakizaka H, Okada M, Odaka K, Yui J, Tsuji AB, Fukumura T, Zhang MR (2014) OAT3-mediated extrusion of the 99mTc-ECD metabolite in the mouse brain. J Cereb Blood Flow Metab 34:585–588
Asaba H, Hosoya K, Takanaga H, Ohtsuki S, Tamura E, Takizawa T, Terasaki T (2000) Blood–brain barrier is involved in the efflux transport of a neuroactive steroid, dehydroepiandrosterone sulfate, via organic anion transporting polypeptide 2. J Neurochem 75:1907–1916
Gao B, Stieger B, Noe B, Fritschy JM, Meier PJ (1999) Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem 47:1255–1264
Sugiyama D, Kusuhara H, Shitara Y, Abe T, Meier PJ, Sekine T, Endou H, Suzuki H, Sugiyama Y (2001) Characterization of the efflux transport of 17beta-estradiol-D-17beta-glucuronide from the brain across the blood–brain barrier. J Pharmacol Exp Ther 298:316–322
Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ (2000) Organic anion-transporting polypeptides mediate transport of opioid peptides across blood–brain barrier. J Pharmacol Exp Ther 294:73–79
Guo Y, Jiang L (2016) Organic anion transporting polypeptide 2 transports valproic acid in rat brain microvascular endothelial cells. Neurol Res 38:634–639
Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y (2003) Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood–brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495
Mayerl S, Muller J, Bauer R, Richert S, Kassmann CM, Darras VM, Buder K, Boelen A, Visser TJ, Heuer H (2014) Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest 124:1987–1999
Lin CJ, Tai Y, Huang MT, Tsai YF, Hsu HJ, Tzen KY, Liou HH (2010) Cellular localization of the organic cation transporters, OCT1 and OCT2, in brain microvessel endothelial cells and its implication for MPTP transport across the blood–brain barrier and MPTP-induced dopaminergic toxicity in rodents. J Neurochem 114:717–727
Wu KC, Lu YH, Peng YH, Tsai TF, Kao YH, Yang HT, Lin CJ (2015) Decreased expression of organic cation transporters, Oct1 and Oct2, in brain microvessels and its implication to MPTP-induced dopaminergic toxicity in aged mice. J Cereb Blood Flow Metab 35:37–47
Busch AE, Quester S, Ulzheimer JC, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H (1996) Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J Biol Chem 271:32599–32604
Kido Y, Tamai I, Ohnari A, Sai Y, Kagami T, Nezu J, Nikaido H, Hashimoto N, Asano M, Tsuji A (2001) Functional relevance of carnitine transporter OCTN2 to brain distribution of l-carnitine and acetyl-l-carnitine across the blood–brain barrier. J Neurochem 79:959–969
Miecz D, Januszewicz E, Czeredys M, Hinton BT, Berezowski V, Cecchelli R, Nalecz KA (2008) Localization of organic cation/carnitine transporter (OCTN2) in cells forming the blood–brain barrier. J Neurochem 104:113–123
Friedrich A, Prasad PD, Freyer D, Ganapathy V, Brust P (2003) Molecular cloning and functional characterization of the OCTN2 transporter at the RBE4 cells, an in vitro model of the blood–brain barrier. Brain Res 968:69–79
Okura T, Kato S, Deguchi Y (2014) Functional expression of organic cation/carnitine transporter 2 (OCTN2/SLC22A5) in human brain capillary endothelial cell line hCMEC/D3, a human blood–brain barrier model. Drug Metab Pharmacokinet 29:69–74
Wu X, Prasad PD, Leibach FH, Ganapathy V (1998) cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochem Biophys Res Commun 246:589–595
Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A (1998) Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem 273:20378–20382
Czeredys M, Samluk L, Michalec K, Tulodziecka K, Skowronek K, Nalecz KA (2013) Caveolin-1–a novel interacting partner of organic cation/carnitine transporter (Octn2): effect of protein kinase C on this interaction in rat astrocytes. PLoS One 8:e82105
Shinawi M, Gruener N, Lerner A (1998) CSF levels of carnitine in children with meningitis, neurologic disorders, acute gastroenteritis, and seizure. Neurology 50:1869–1871
Bennett KM, Liu J, Hoelting C, Stoll J (2011) Expression and analysis of two novel rat organic cation transporter homologs, SLC22A17 and SLC22A23. Mol Cell Biochem 352:143–154
Miyamoto T, Asaka R, Suzuki A, Takatsu A, Kashima H, Shiozawa T (2011) Immunohistochemical detection of a specific receptor for lipocalin2 (solute carrier family 22 member 17, SLC22A17) and its prognostic significance in endometrial carcinoma. Exp Mol Pathol 91:563–568
Serrano Leon A, Amir Shaghaghi M, Yurkova N, Bernstein CN, El-Gabalawy H, Eck P (2014) Single-nucleotide polymorphisms in SLC22A23 are associated with ulcerative colitis in a Canadian white cohort. Am J Clin Nutr 100:289–294
Okura T, Higuchi K, Kitamura A, Deguchi Y (2014) Proton-coupled organic cation antiporter-mediated uptake of apomorphine enantiomers in human brain capillary endothelial cell line hCMEC/D3. Biol Pharm Bull 37:286–291
Mehta DC, Short JL, Nicolazzo JA (2013) Memantine transport across the mouse blood–brain barrier is mediated by a cationic influx H+ antiporter. Mol Pharm 10:4491–4498
Carvey PM, Hendey B, Monahan AJ (2009) The blood–brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem 111:291–314
Prasad S, Sajja RK, Naik P, Cucullo L (2014) Diabetes mellitus and blood–brain barrier dysfunction: an overview. J Pharmacovigil 2:125
Acknowledgments
Part of this manuscript quotes the results obtained by my team during realization of Grants 4427/B/P01/2010/38 and 2012/07/B/NZ3/00225 financed by the National Science Centre in Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflict of interest.
Additional information
This work is dedicated to Prof. Jan Albrecht with acknowledgements for his contribution to science and scientific society.
Rights and permissions
About this article
Cite this article
Nałęcz, K.A. Solute Carriers in the Blood–Brain Barier: Safety in Abundance. Neurochem Res 42, 795–809 (2017). https://doi.org/10.1007/s11064-016-2030-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2030-x