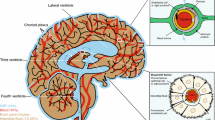
Abstract
The brain exchanges nutrients and small molecules with blood via the blood–brain barrier (BBB). Approximately 20% energy intake for the body is consumed by the brain. Glucose is known for its critical roles for energy production and provides substrates for biogenesis in neurons. The brain takes up glucose via glucose transporters GLUT1 and 3, which are expressed in several neural cell types. The brain is also equipped with various transport systems for acquiring amino acids, lactate, ketone bodies, lipids, and cofactors for neuronal functions. Unraveling the mechanisms by which the brain takes up and metabolizes these nutrients will be key in understanding the nutritional requirements in the brain. This could also offer opportunities for therapeutic interventions in several neurological disorders. For instance, emerging evidence suggests a critical role of lactate as an alternative energy source for neurons. Neuronal cells express monocarboxylic transporters to acquire lactate. As such, treatment of GLUT1-deficient patients with ketogenic diets to provide the brain with alternative sources of energy has been shown to improve the health of the patients. Many transporters are present in the brain, but only a small number has been characterized. In this review, we will discuss about the roles of solute carrier (SLC) transporters at the blood brain barrier (BBB) and neural cells, in transport of nutrients and metabolites in the brain.

Similar content being viewed by others
Availability of data and material
Not applicable.
Abbreviations
- ABC transporter:
-
ATP-binding cassette transporter
- AD:
-
Alzheimer’s disease
- ADHD:
-
Attention-deficit/hyperactivity disorder
- AHDS:
-
Allan–Herndon–Dudley syndrome
- Akt:
-
Serine/threonine protein kinase
- APP:
-
Amyloid precursor protein
- APP/PS1:
-
APPswe/PS1dE9
- Aß peptide:
-
ß-amyloid peptide
- ASD:
-
Autism spectrum disorders
- ASYMAD:
-
Asymptomatic AD
- ATP:
-
Adenosine triphosphate
- Aβ:
-
Amyloid β-peptide
- BBB:
-
Blood–brain barrier
- CD31:
-
Cluster of differentiation 31
- CD36:
-
Cluster of differentiation 36
- CD4:
-
Cluster of differentiation 4
- CNS:
-
Central nervous system
- CNV:
-
Copy number variation
- CREB:
-
CAMP-response element
- D2:
-
Type 2 deiodinase
- DHA:
-
Docosahexaenoic acid
- EAAT:
-
Excitatory amino acid transporters
- ECs:
-
Endothelial cells
- ED:
-
Epileptiform discharges
- EEG:
-
Electroencephalography
- EGF:
-
Epidermal growth factor
- EMT:
-
Extra-neuronal monoamine transporter
- ENU:
-
N-Ethyl-N-nitrosourea
- FAD:
-
Flavin adenine dinucleotide
- FAs:
-
Fatty acids
- FAT:
-
Fatty acid translocase
- FATP:
-
Fatty acid transport proteins
- FMN:
-
Flavin mononucleotide
- GAA:
-
Guanidinoacetate
- GBM:
-
Glioblastoma
- GLUT:
-
Glucose transporter
- GLUT1-DS:
-
GLUT1 deficiency
- GLUT1-DS:
-
GLUT1 deficiency syndrome
- GSCs:
-
GBM cancer stem cells
- GT1AS:
-
Antisense-GLUT1 cDNA
- hCMEC/D3:
-
Human brain endothelial cells
- His:
-
Histidine
- HMGA1:
-
The high mobility Group A1
- HRE:
-
Hypoxia-responsive element
- HTLV:
-
Human T-lymphotropic virus
- IGF-1:
-
Insulin-like growth factor 1
- Ile:
-
Isoleucine
- KO:
-
Knock-out
- LAT:
-
L amino acid transporter
- LDHB:
-
Lactate dehydrogenase B
- Leu:
-
Leucine
- LRP1:
-
Low-density lipoprotein receptor-related protein 1
- MCTs:
-
Monocarboxylate transporters
- Met:
-
Methionine
- MFS:
-
Major facilitator superfamily
- Mfsd2a:
-
Major facilitator superfamily domain-containing protein 2a
- mTOR:
-
Mammalian target of rapamycin
- N- and C-termini:
-
Amino and carboxyl termini
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NFTs:
-
Neurofibrillary tangles
- Oatp1c1:
-
Organic anion transporter polypeptide 1c1
- OCTN:
-
Organic cation novel transporter
- PEST domains:
-
Domains enriched of Pro, Glu, Ser, Thr
- Phe:
-
Phenylalanine
- PI3K:
-
Phosphatidylinositol 3-kinase
- PKC:
-
Protein kinase C
- RA:
-
Rheumatoid arthritis
- RFVT1:
-
Riboflavin transporter 1
- RFVT2:
-
Riboflavin transporter 2
- RFVT3:
-
Riboflavin transporter 3
- ROS:
-
Reactive oxygen species
- SLC16:
-
Solute carrier 16
- SLC52:
-
Solute carrier family 52
- SVCT:
-
Sodium-dependent vitamin C transporter
- T3 :
-
3,5,3′-Triiodothyronine
- T4 :
-
Thyroxine
- Thr:
-
Threonine
- TLS:
-
Translation start site
- Trp:
-
Tryptophan
- TRs:
-
Thyroid hormone receptors
- TSC1/2:
-
Tuberous sclerosis proteins 1/2
- TXNIP:
-
Identification of thioredoxin-interacting protein
- Tyr:
-
Tyrosine
- UDP-glucose:
-
Uracil-diphosphate glucose
- Val:
-
Valine
- VE-Cadherin:
-
Vascular endothelial (VE)-cadherin
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- Wnt:
-
Wingless-related integration site
References
Mergenthaler P et al (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36(10):587–597
Watts ME, Pocock R, Claudianos C (2018) Brain energy and oxygen metabolism: emerging role in normal function and disease. Front Mol Neurosci 11:216
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14(3):133–150
Vanlandewijck M et al (2018) A molecular atlas of cell types and zonation in the brain vasculature. Nature 554(7693):475–480
Lauritzen KH et al (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24(10):2784–2795
Bergersen LH, Gjedde A (2012) Is lactate a volume transmitter of metabolic states of the brain? Front Neuroenergetics 4:5–5
Veech RL (1991) The metabolism of lactate. NMR Biomed 4:53–58
Dienel GA (2012) Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 32(7):1107–1138
Cataldo AM, Broadwell RD (1986) Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions: I. Neurons and glia. J Electron Microsc Tech 3(4):413–437
Dienel GA, Cruz NF (2015) Contributions of glycogen to astrocytic energetics during brain activation. Metab Brain Dis 30(1):281–298
Suzuki A et al (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144(5):810–823
Poitelon Y, Kopec AM, Belin S (2020) Myelin fat facts: an overview of lipids and fatty acid metabolism. Cells 9(4):812–812
Wakil SJ, Stoops JK, Joshi VC (1983) fatty acid synthesis and its regulation. Annu Rev Biochem 52(1):537–579
Röder PV et al (2016) Pancreatic regulation of glucose homeostasis. Exp Mol Med 48(3):e219–e219
Ritter S (2017) Monitoring and maintenance of brain glucose supply: importance of hindbrain catecholamine neurons in this multifaceted task. In: Harris RBS (ed) BTI—appetite and food intake: central control, in appetite and food intake: central control, 2nd edn. CRC Press, Boca Raton
Meierhans R et al (2010) Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit Care (London, England) 14(1):R13–R13
Wright EM, Loo DD, Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91(2):733–794
Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34(2–3):121–138
Holman GD (2020) Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflügers Arch Eur J Physiol 472(9):1155–1175
Gorovits N, Charron MJ (2003) What we know about facilitative glucose transporters: lessons from cultured cells, animal models, and human studies. Biochem Mol Biol Educ 31(3):163–172
Harvey Lodish AB, Lawrence Z, Paul M, David B, James D (2000) Section 15.3: uniporter-catalyzed transport. In: molecular cell biology, 4th edn. W H Freeman & Co (Sd), New York
Deng D et al (2014) Crystal structure of the human glucose transporter GLUT1. Nature 510(7503):121–125
Sun Y et al (2019) sgRNA-shRNA structure mediated SNP site editing on porcine IGF2 gene by CRISPR/StCas9. Front Genet 10:347
Devraj K et al (2011) GLUT-1 glucose transporters in the blood-brain barrier: differential phosphorylation. J Neurosci Res 89(12):1913–1925
Duelli R, Kuschinsky W (2001) Brain glucose transporters: relationship to local energy demand. Physiology 16(2):71–76
Simpson IA et al (2008) The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab 295(2):E242–E253
Maher F, Simpson IA (1994) The GLUT3 glucose transporter is the predominant isoform in primary cultured neurons: assessment by biosynthetic and photoaffinity labelling. Biochem J 301((Pt 2)):379–384
Messana T et al (2018) Glucose transporter type 1 deficiency syndrome: developmental delay and early-onset ataxia in a novel mutation of the SLC2A1 gene. J Pediatr Neurosci 13(4):496–499
Asano T et al (1991) The role of N-glycosylation of GLUT1 for glucose transport activity. J Biol Chem 266(36):24632–24636
Thorens B, Mueckler M (2010) Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab 298(2):E141–E145
Korotcov AV et al (2012) Glucosamine-linked near-infrared fluorescent probes for imaging of solid tumor xenografts. Mol Imag Biol 14(4):443–451
Wang Y-Z et al (2018) Brain transport profiles of Ginsenoside Rb1 by glucose transporter 1: in vitro and in vivo. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00398
Kasahara T et al (2009) Identification of a key residue determining substrate affinity in the human glucose transporter GLUT1. Biochim Biophys Acta (BBA) 1788(5):1051–1055
Kapoor K et al (2016) Mechanism of inhibition of human glucose transporter GLUT1 is conserved between cytochalasin B and phenylalanine amides. Proc Natl Acad Sci 113(17):4711
Siska PJ, Rathmell JC (2015) PKCs sweeten cell metabolism by phosphorylation of Glut1. Mol Cell 58(5):711–712
Nasoohi S, Ismael S, Ishrat T (2018) Thioredoxin-interacting protein (TXNIP) in cerebrovascular and neurodegenerative diseases: regulation and implication. Mol Neurobiol 55(10):7900–7920
Wang D et al (2006) A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet 15(7):1169–1179
Heilig CW et al (2003) Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc Natl Acad Sci USA 100(26):15613–15618
Furuse T et al (2019) A new mouse model of GLUT1 deficiency syndrome exhibits abnormal sleep-wake patterns and alterations of glucose kinetics in the brain. Disease Models Mech 12(9):dmm038828
Zheng P-P et al (2010) Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann Neurol 68(6):835–844
Tang M et al (2017) Brain microvasculature defects and Glut1 deficiency syndrome averted by early repletion of the glucose transporter-1 protein. Nat Commun 8:14152–14152
Vaudano AE et al (2017) Brain correlates of spike and wave discharges in GLUT1 deficiency syndrome. Neuroimage Clin 13:446–454
Sánchez Fernández I et al (2015) Should epileptiform discharges be treated? Epilepsia 56(10):1492–1504
Aldenkamp AP, Arends J (2004) Effects of epileptiform EEG discharges on cognitive function: is the concept of “transient cognitive impairment” still valid? Epilepsy Behav 5(Suppl 1):S25-34
Veys K et al (2020) Role of the glut1 glucose transporter in postnatal CNS angiogenesis and blood–brain barrier integrity. Circ Res 127(4):466–482
Ziegler GC et al (2020) Cellular effects and clinical implications of SLC2A3 copy number variation. J Cell Physiol 235(12):9021–9036
Yano H et al (1991) Tissue distribution and species difference of the brain type glucose transporter (GLUT3). Biochem Biophys Res Commun 174(2):470–477
Leino RL et al (1997) Ultrastructural localization of GLUT 1 and GLUT 3 glucose transporters in rat brain. J Neurosci Res 49(5):617–626
Rumsey S et al (1997) Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem 272:18982–18989
Burant CF, Bell GI (1992) Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry 31(42):10414–10420
Heilig C et al (2003) Implications of glucose transporter protein type 1 (GLUT1)-haplodeficiency in embryonic stem cells for their survival in response to hypoxic stress. Am J Pathol 163(5):1873–1885
Patching SG (2017) Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol 54(2):1046–1077
Ganguly A et al (2007) Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab 292(5):E1241–E1255
Schmidt S et al (2008) Neuronal functions, feeding behavior, and energy balance in Slc2a3+/− mice. Am J Physiol Endocrinol Metab 295(5):E1084–E1094
Shin B-C et al (2018) Neural deletion of glucose transporter isoform 3 creates distinct postnatal and adult neurobehavioral phenotypes. J Neurosci 38(44):9579–9599
Stuart CA et al (2011) Brain glucose transporter (Glut3) haploinsufficiency does not impair mouse brain glucose uptake. Brain Res 1384:15–22
Arsov T et al (2012) Early onset absence epilepsy: 1 in 10 cases is caused by GLUT1 deficiency. Epilepsia 53(12):e204–e207
Rotstein M et al (2010) Glut1 deficiency: inheritance pattern determined by haploinsufficiency. Ann Neurol 68(6):955–958
Wang D et al (2005) Glut-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann Neurol 57(1):111–118
Klepper J et al (2020) Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open 5(3):354–365
Pascual JM et al (2014) Triheptanoin for glucose transporter type I deficiency (G1D): modulation of human ictogenesis, cerebral metabolic rate, and cognitive indices by a food supplement. JAMA Neurol 71(10):1255–1265
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25(1):59–70
Murphy MP, LeVine H 3rd (2010) Alzheimer’s disease and the amyloid-beta peptide. J Alzheimer’s Dis 19(1):311–323
Lee HJ et al (2018) Diabetes and Alzheimer’s disease: mechanisms and nutritional aspects. Clin Nutr Res 7(4):229–240
Dineley KT, Jahrling JB, Denner L (2014) Insulin resistance in Alzheimer’s disease. Neurobiol Dis 72(Pt A):92–103
Cholerton B et al (2016) Type 2 diabetes, cognition, and dementia in older adults: toward a precision health approach. Diabetes Spectr 29(4):210–219
Winkler EA et al (2015) GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 18(4):521–530
Ramanathan A et al (2015) Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: the role, regulation and restoration of LRP1. Front Aging Neurosci 7:136
Zlokovic BV et al (2010) Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem 115(5):1077–1089
Zhao Z et al (2015) Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci 18(7):978–987
Donahue JE et al (2006) RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol 112(4):405–415
Driscoll I, Troncoso J (2011) Asymptomatic Alzheimer’s disease: a prodrome or a state of resilience? Curr Alzheimer Res 8(4):330–335
Liu Y et al (2009) Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J Neurochem 111(1):242–249
Flavahan WA et al (2013) Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci 16(10):1373–1382
Kuang R et al (2017) GLUT3 upregulation promotes metabolic reprogramming associated with antiangiogenic therapy resistance. JCI Insight 2(2):e88815
Kuo M-H et al (2019) Glucose transporter 3 is essential for the survival of breast cancer cells in the brain. Cells 8(12):1568
Yu J et al (2012) IGF-1 induces hypoxia-inducible factor 1α-mediated GLUT3 expression through PI3K/Akt/mTOR dependent pathways in PC12 cells. Brain Res 1430:18–24
Zhang Z et al (2018) PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol Med Rep 18(4):3547–3554
Libby CJ et al (2018) Identification of compounds that decrease glioblastoma growth and glucose uptake in vitro. ACS Chem Biol 13(8):2048–2057
Halestrap AP, Meredith D (2004) The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447(5):619–628
Halestrap AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343(Pt 2):281–299
Halestrap AP (2013) The SLC16 gene family—structure, role and regulation in health and disease. Mol Aspects Med 34(2–3):337–349
Murakami Y et al (2005) Functional characterization of human monocarboxylate transporter 6 (SLC16A5). Drug Metab Dispos 33(12):1845–1851
Jones RS et al (2019) Characterization and proteomic-transcriptomic investigation of monocarboxylate transporter 6 knockout mice: evidence of a potential role in glucose and lipid metabolism. Mol Pharmacol 96(3):364–376
Dai W et al (2020) Genetic variants in PDSS1 and SLC16A6 of the ketone body metabolic pathway predict cutaneous melanoma-specific survival. Mol Carcinog 59(6):640–650
Karanth S, Schlegel A (2018) The monocarboxylate transporter SLC16A6 regulates adult length in zebrafish and is associated with height in humans. Front Physiol 9:1936
Friesema EC et al (2003) Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278(41):40128–40135
Friesema EC et al (2008) Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol 22(6):1357–1369
Suhre K et al (2011) Human metabolic individuality in biomedical and pharmaceutical research. Nature 477(7362):54–60
Jansen L et al (2014) HBsAg loss in patients treated with peginterferon alfa-2a and adefovir is associated with SLC16A9 gene variation and lower plasma carnitine levels. J Hepatol 61(4):730–737
Almeda-Valdes P et al (2019) The SLC16A11 risk haplotype is associated with decreased insulin action, higher transaminases and large-size adipocytes. Eur J Endocrinol 180(2):99–107
Abplanalp J et al (2013) The cataract and glucosuria associated monocarboxylate transporter MCT12 is a new creatine transporter. Hum Mol Genet 22(16):3218–3226
Jomura R et al (2020) Monocarboxylate transporter 12 as a guanidinoacetate efflux transporter in renal proximal tubular epithelial cells. Biochim Biophys Acta Biomembr 1862(11):183434
Takahashi M et al (2020) Functional characterization of monocarboxylate transporter 12 (SLC16A12/MCT12) as a facilitative creatine transporter. Drug Metab Pharmacokinet 35(3):281–287
Rafiki A et al (2003) Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience 122(3):677–688
Halestrap AP, Wilson MC (2012) The monocarboxylate transporter family–role and regulation. IUBMB Life 64(2):109–119
Gerhart DZ et al (1997) Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol 273(1 Pt 1):E207–E213
Lengacher S et al (2013) Resistance to diet-induced obesity and associated metabolic perturbations in haploinsufficient monocarboxylate transporter 1 mice. PLoS ONE 8(12):e82505
Merezhinskaya N et al (2000) Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve 23(1):90–97
Wang J et al (2019) Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell 25(6):754–767
Liu Z et al (2016) Regulation of monocarboxylic acid transporter 1 trafficking by the canonical Wnt/β-catenin pathway in rat brain endothelial cells requires cross-talk with notch signaling. J Biol Chem 291(15):8059–8069
Tang X et al (2017) Effect of nitric oxide to axonal degeneration in multiple sclerosis via downregulating monocarboxylate transporter 1 in oligodendrocytes. Nitric Oxide 67:75–80
Liu S et al (2017) miR-219 attenuates demyelination in cuprizone-induced demyelinated mice by regulating monocarboxylate transporter 1. Eur J Neurosci 45(2):249–259
Fünfschilling U et al (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485(7399):517–521
Domènech-Estévez E et al (2015) Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J Neurosci 35(10):4151–4156
Jha MK et al (2020) Monocarboxylate transporter 1 in Schwann cells contributes to maintenance of sensory nerve myelination during aging. Glia 68(1):161–177
Morrison BM et al (2015) Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Exp Neurol 263:325–338
Bouçanova, F., et al., Disrupted function of lactate transporter MCT1, but not MCT4, in Schwann cells affects the maintenance of motor end-plate innervation. Glia, 2020.
Pang R et al (2020) The distribution and density of monocarboxylate transporter 2 in cerebral cortex, hippocampus and cerebellum of wild-type mice. J Anat 236(2):370–377
Pierre K, Pellerin L (2005) Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 94(1):1–14
Mason S (2017) Lactate shuttles in neuroenergetics-homeostasis, allostasis and beyond. Front Neurosci 11:43
Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86(3):1009–1031
Netzahualcoyotzi C, Pellerin L (2020) Neuronal and astroglial monocarboxylate transporters play key but distinct roles in hippocampus-dependent learning and memory formation. Prog Neurobiol 194:101888
Serrano-Pozo A et al (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1(1):a006189
Pedros I et al (2016) Molecular links between early energy metabolism alterations and Alzheimer’s disease. Front Biosci (Landmark Ed) 21:8–19
Hong P et al (2020) Role of monocarboxylate transporter 4 in Alzheimer disease. Neurotoxicology 76:191–199
Lok K et al (2013) Characterization of the APP/PS1 mouse model of Alzheimer’s disease in senescence accelerated background. Neurosci Lett 557(Pt B):84–89
Park SJ et al (2018) An overview of MCT1 and MCT4 in GBM: small molecule transporters with large implications. Am J Cancer Res 8(10):1967–1976
Miranda-Gonçalves V et al (2016) Hypoxia-mediated upregulation of MCT1 expression supports the glycolytic phenotype of glioblastomas. Oncotarget 7(29):46335–46353
Lai SW et al (2020) Monocarboxylate transporter 4 regulates glioblastoma motility and monocyte binding ability. Cancers (Basel) 12(2):380
Miranda-Gonçalves V et al (2017) Monocarboxylate transporter 1 is a key player in glioma-endothelial cell crosstalk. Mol Carcinog 56(12):2630–2642
Takada T, Takata K, Ashihara E (2016) Inhibition of monocarboxylate transporter 1 suppresses the proliferation of glioblastoma stem cells. J Physiol Sci 66(5):387–396
Lim KS et al (2014) Inhibition of monocarboxylate transporter-4 depletes stem-like glioblastoma cells and inhibits HIF transcriptional response in a lactate-independent manner. Oncogene 33(35):4433–4441
Ganguli M et al (1996) Association between dementia and elevated TSH: a community-based study. Biol Psychiatry 40(8):714–725
Joffe RT, Sokolov ST (1994) Thyroid hormones, the brain, and affective disorders. Crit Rev Neurobiol 8(1–2):45–63
Scanlan TS et al (2004) 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med 10(6):638–642
Friesema EC et al (2006) Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol Endocrinol 20(11):2761–2772
Visser WE et al (2008) Thyroid hormone transport in and out of cells. Trends Endocrinol Metab 19(2):50–56
Mariotta L et al (2012) T-type amino acid transporter TAT1 (Slc16a10) is essential for extracellular aromatic amino acid homeostasis control. J Physiol 590(24):6413–6424
Schwartz CE et al (2005) Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 77(1):41–53
Remerand G et al (2019) Expanding the phenotypic spectrum of Allan-Herndon-Dudley syndrome in patients with SLC16A2 mutations. Dev Med Child Neurol 61(12):1439–1447
López-Espíndola D et al (2014) Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J Clin Endocrinol Metab 99(12):E2799–E2804
Bernal J (2006) Role of monocarboxylate anion transporter 8 (MCT8) in thyroid hormone transport: answers from mice. Endocrinology 147(9):4034–4035
Trajkovic M et al (2007) Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117(3):627–635
Mayerl S et al (2014) Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest 124(5):1987–1999
Mayerl S et al (2012) Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology 153(3):1528–1537
van Mullem AA et al (2016) Effects of thyroid hormone transporters MCT8 and MCT10 on nuclear activity of T3. Mol Cell Endocrinol 437:252–260
Betz AL et al (1994) Blood-brain barrier permeability and brain concentration of sodium, potassium, and chloride during focal ischemia. J Cereb Blood Flow Metab 14(1):29–37
Hawkins RA et al (2013) Synergism between the two membranes of the blood–brain barrier: glucose and amino acid transport. Am J Neurosci Res 1:1–25
Nalecz KA (2017) Solute Carriers in the Blood-Brain Barier: Safety in Abundance. Neurochem Res 42(3):795–809
Sanchez del Pino MM, Peterson DR, Hawkins RA (1995) Neutral amino acid transport characterization of isolated: luminal and abluminal membranes of the blood-brain barrier. J Biol Chem 270(25):14913–14918
Sánchez-Campillo M et al (2019) Decreased blood level of MFSD2a as a potential biomarker of Alzheimer’s disease. Int J Mol Sci 21(1):70–70
Duelli R et al (2000) Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab 20(11):1557–1562
Kanai Y et al (1998) Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem 273(37):23629–23632
Nakamura E et al (1999) 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem 274(5):3009–3016
Prasad, P.D., et al., Human LAT1, a Subunit of System L Amino Acid Transporter: Molecular Cloning and Transport Function 1. 1999.
Segawa H et al (1999) Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity*. J Biol Chem. https://doi.org/10.1074/jbc.274.28.19745
Kanai Y et al (1998) Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98)*. J Biol Chem. https://doi.org/10.1074/jbc.273.37.23629
Mastroberardino L et al (1998) Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. https://doi.org/10.1038/26246
Meier C (2002) Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J 21(4):580–589
Geier EG et al (2013) Structure-based ligand discovery for the large-neutral amino acid transporter 1 LAT-1. Proc Natl Acad Sci USA 110:5480–5485
Napolitano L et al (2017) Novel insights into the transport mechanism of the human amino acid transporter LAT1 (SLC7A5) Probing critical residues for substrate translocation. Biochim Biophys Acta. https://doi.org/10.1016/j.bbagen.2017.01.013
Fotiadis D, Kanai Y, Palacín M (2013) The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34:139–158
Singh N, Ecker GF (2018) Insights into the structure, function, and ligand discovery of the large neutral amino acid transporter 1, lat1. Int J Mol Sci 19:1278
Tărlungeanu DC et al (2016) Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell 167(6):1481–1494
Poncet N et al (2014) LAT1 (Slc7a5) null mice are embryonic lethal and exhibit neural tube defects (8965). FASEB J 28(S1):896
Ohtsuki S et al (2010) Reduction of L-type amino acid transporter 1 mRNA expression in brain capillaries in a mouse model of Parkinson’s disease. Biol Pharm Bull 33:1250–1252
Stoll J, Wadhwani KC, Smith QR (1993) Identification of the cationic amino acid transporter (System y+) of the rat blood–brain barrier. J Neurochem 60(5):1956–1959
Closs EI et al (2006) Structure and function of cationic amino acid transporters (CATs). Springer, Berlin, pp 67–77
Devés R, Boyd CAR (1989) Transporters for cationic amino acids in animal cells: discovery, structure, and function. American Physiological Society, Rockville, pp 487–545
Wang H, Kavanaugh MP, Kabat D (1994) A critical site in the cell surface receptor for ecotropic murine retroviruses required for amino acid transport but not for viral reception. Virology 202(2):1058–1060
O’Kane RL et al (2006) Cationic amino acid transport across the blood-brain barrier is mediated exclusively by system y+. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/ajpendo.00007.2006291(2)
Perkins CP et al (1997) Anemia and perinatal death result from loss of the murine ecotropic retrovirus receptor mCAT-1. Genes Dev 11(7):914–925
Bridges RJ, Natale NR, Patel SA (2012) System x c-cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Wiley, New York, pp 20–34
Conrad M, Sato H (2012) The oxidative stress-inducible cystine/glutamate antiporter, system x c-: cystine supplier and beyond. Springer, Berlin
Sato H et al (1999) Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274(17):11455–11458
Fagerberg L et al (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 13(2):397–406
Burdo J, Dargusch R, Schubert D (2006) Distribution of the cystine/glutamate antiporter system xc- in the brain, kidney, and duodenum. J Histochem Cytochem 54(5):549–557
Bannai S et al (1986) Induction of cystine transport activity in isolated rat hepatocytes by sulfobromophthalein and other electrophilic agents. Hepatology 6(6):1361–1368
Watanabe H, Bannai S (1987) Induction of cystine transport activity in mouse peritoneal macrophages. J Exp Med 165(3):628–640
Nabeyama A et al (2010) xCT deficiency accelerates chemically induced tumorigenesis. Proc Natl Acad Sci USA 107(14):6436–6441
de Bundel D et al (2011) Loss of system xc- does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. J Neurosci 31(15):5792–5803
Zaragozá R (2020) Transport of amino acids across the blood-brain barrier. Front Physiol 11:973–973
Takanaga H et al (2005) Characterization of a branched-chain amino-acid transporter SBAT1 (SLC6A15) that is expressed in human brain. Biochem Biophys Res Commun 337(3):892–900
Melikian HE (2004) Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther 104(1):17–27
Sakai R et al (2004) Leucine-nitrogen metabolism in the brain of conscious rats: its role as a nitrogen carrier in glutamate synthesis in glial and neuronal metabolic compartments. J Neurochem 88(3):612–622
Drgonova J et al (2007) Deletion of v7–3 (SLC6A15) transporter allows assessment of its roles in synaptosomal proline uptake, leucine uptake and behaviors. Brain Res 1183(1):10–20
Hägglund MGA et al (2013) B0AT2 (SLC6A15) is localized to neurons and astrocytes, and is involved in mediating the effect of leucine in the brain. PLoS ONE 8(3):e58651
Nałęcz KA (2017) solute carriers in the blood–brain barier: safety in abundance. Neurochem Res 42(3):795–809
Hatanaka T et al (2000) Primary structure, functional characteristics and tissue expression pattern of human ATA2, a subtype of amino acid transport system A. Biochim Biophys Acta 1467(1):1–6
Lee WJ et al (1998) Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am J Physiol 274:43–44
Menchini RJ, Chaudhry FA (2019) Multifaceted regulation of the system A transporter Slc38a2 suggests nanoscale regulation of amino acid metabolism and cellular signaling. Elsevier, Amsterdam, p 107789
Ruderisch N et al (2011) Differential axial localization along the mouse brain vascular tree of luminal sodium-dependent glutamine transporters Snat1 and Snat3. J Cereb Blood Flow Metab 31(7):1637–1647
O’Kane RL et al (2004) Na+-dependent neutral amino acid transporters A, ASC, and N of the blood-brain barrier: mechanisms for neutral amino acid removal. Am J Physiol Endocrinol Metab 287(4):E622–E629
Yamada D et al (2019) Inhibition of the glutamine transporter SNAT1 confers neuroprotection in mice by modulating the mTOR-autophagy system. Commun Biol 2(1):1–11
Papadakis M et al (2013) Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat Med 19(3):351–357
Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nature Publishing Group, Berlin, pp 983–997
Nakajo T et al (2004) Glutamine is a key regulator for amino acid-controlled cell growth through the mTOR signaling pathway in rat intestinal epithelial cells. Biochem Biophys Res Commun 326(1):174–180
O’Kane RL et al (1999) Na+-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem 274(45):31891–31895
Hans Christian Cederberg H, Carsten Uhd N, Brodin B (2014) Glutamate efflux at the blood-brain barrier: cellular mechanisms and potential clinical relevance. Elsevier, Amsterdam, pp 639–645
Jl A et al (1994) Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 14(9):5559–5569
O’Kane RL, Hawkins RA (2003) Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood–brain barrier. Am J Physiol Endocrinol Metab 285:48–66
O’Donovan SM, Sullivan CR, McCullumsmith RE (2017) The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr 3(1):32–32
Chan JP et al (2018) The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol 16(8):1–30
Parkin GM et al (2018) Glutamate transporters, EAAT1 and EAAT2, are potentially important in the pathophysiology and treatment of schizophrenia and affective disorders. World J Psychiatry 8(2):51–51
O’Brien JS, Fillerup DL, Mead JF (1964) Brain lipids: I. Quantification and fatty acid composition of. J Lipid Res 15:109–116
Aa F et al (2007) Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res Rev 56(2):443–471
Hansen HS, Artmann A (2008) Endocannabinoids and Nutrition. J Neuroendocrinol 20(Suppl 1):94–99
Tan ST et al (2020) (2020) Emerging roles of lysophospholipids in health and disease. Prog Lipid Res 80:101068
Nguyen LN et al (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509(7501):503–506
Ben-Zvi A et al (2014) Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 509:507–511
Quek DQY et al (2016) Structural insights into the transport mechanism of the human sodium-dependent lysophosphatidylcholine transporter MFSD2A. J Biol Chem 291(18):9383–9394
Cater RJ et al (2021) Structural basis of omega-3 fatty acid transport across the blood–brain barrier. Nature 595:315–319
Andreone BJ et al (2017) Blood–brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. https://doi.org/10.1016/j.neuron.2017.03.043
Andaloussi Mae M et al (2020) Single-cell analysis of blood–brain barrier response to pericyte loss. Circ Res. https://doi.org/10.1161/CIRCRESAHA.120.317473
Wong BH et al (2016) mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid (DHA) in eye and is important for photoreceptor cell development. J Biol Chem 291(20):10501–10514
Alakbarzade V et al (2015) A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet 47(7):814–817
Guemez-Gamboa A et al (2015) Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet 47:809–813
Harel T et al (2018) Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination. Neurogenetics 19(4):227–235
Sugasini D et al (2017) Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci Rep 7(1):1–11
Brenna JT, Carlson SE (2014) Docosahexaenoic acid and human brain development: evidence that adietary supply is needed for optimal development. J Hum Evol 77:99–106
Olson CR, Mello CV (2010) Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res 54(4):489–495
Zhang J et al (2021) Vitamin E deficiency dysregulates thiols, amino acids and related molecules during zebrafish embryogenesis. Redox Biol 38:101784
Zhong M et al (2013) Vitamin A transport and the transmembrane pore in the cell-surface receptor for plasma retinol binding protein. PLoS ONE 8(11):e73838
Chen Y et al (2016) Structure of the STRA6 receptor for retinol uptake. Science 353:6302
Kelly M et al (2016) Transport of vitamin A across blood-tissue barriers is facilitated by STRA6. FASEB J 30(8):2985–2995
Savini I et al (2008) SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34(3):347–355
Agus DB et al (1997) Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest 100(11):2842–2848
Harrison FE, May JM (2009) Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med 46(6):719–730
Bornstein SR et al (2003) Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2). FASEB J 17(13):1928–1930
Harrison FE et al (2010) Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic Biol Med 49(5):821–829
Covarrubias-Pinto A et al (2020) Impaired intracellular trafficking of sodium-dependent vitamin C transporter 2 contributes to the redox imbalance in Huntington’s disease. J Neurosci Res 38:178–188
Dhir S et al (2019) Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front Psychiatry 10:207
Manzetti S, Zhang J, van der Spoel D (2014) Thiamin function, metabolism, uptake, and transport. Biochemistry 53(5):821–835
Greenwood J, Love ER, Pratt OE (1982) Kinetics of thiamine transport across the blood-brain barrier in the rat. J Physiol 327:95–103
Patel M et al (2012) Molecular and functional characterization of riboflavin specific transport system in rat brain capillary endothelial cells. Brain Res 1468:1–10
Mosegaard S et al (2017) An intronic variation in SLC52A1 causes exon skipping and transient riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Mol Genet Metab 122(4):182–188
Yao Y et al (2010) Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J Nutr 140(7):1220–1226
Berezowski V et al (2004) Involvement of OCTN2 and B0,+ in the transport of carnitine through an in vitro model of the blood–brain barrier. J Neurochem 91(4):860–872
Michalec K et al (2014) Protein kinase C restricts transport of carnitine by amino acid transporter ATB(0,+) apically localized in the blood-brain barrier. Arch Biochem Biophys 554:28–35
Czeredys M et al (2008) A polarized localization of amino acid/carnitine transporter B(0,+) (ATB(0,+)) in the blood–brain barrier. Biochem Biophys Res Commun 376(2):267–270
Okura T, Kato S, Deguchi Y (2014) Functional expression of organic cation/carnitine transporter 2 (OCTN2/SLC22A5) in human brain capillary endothelial cell line hCMEC/D3, a human blood-brain barrier model. Drug Metab Pharmacokinet 29(1):69–74
Juraszek B, Czarnecka-Herok J, Nałęcz KA (2020) Glioma cells survival depends both on fatty acid oxidation and on functional carnitine transport by SLC22A5. J Neurochem 156:642–657
Tucker RAJ, Cheah IK, Halliwell B (2019) Specificity of the ergothioneine transporter natively expressed in HeLa cells. Biochem Biophys Res Commun 513(1):22–27
Koh SS et al (2021) Effect of ergothioneine on 7-ketocholesterol-induced endothelial injury. Neuromolecular Med 23(1):184–198
Kalailingam P et al (2020) Deficiency of MFSD7c results in microcephaly-associated vasculopathy in Fowler syndrome. J Clin Invest 130(8):4081–4093
Acknowledgements
This study was supported in part by Singapore Ministry of Health’s National Research Council NMRC/OFIRG/0066/20, Ministry of Education MOE2018-T2-1-126, MOE-Tier-1, and NUSMED-FOS Joint Research Programme grant on Healthy Brain Aging grants (to L.N.N.).
Funding
Government funding sources. Singapore Ministry of Health’s National Research Council NMRC/OFIRG/0066/20, Ministry of Education MOE2018-T2-1–126, MOE-Tier-1, and NUSMED-FOS Joint Research Programme grant on Healthy Brain Aging grants.
Author information
Authors and Affiliations
Contributions
YTKN, HTTH, THN drafted the manuscript. LNN edited and proofreading of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Applicable. The authors approve for publication of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, Y.T.K., Ha, H.T.T., Nguyen, T.H. et al. The role of SLC transporters for brain health and disease. Cell. Mol. Life Sci. 79, 20 (2022). https://doi.org/10.1007/s00018-021-04074-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-021-04074-4