Abstract
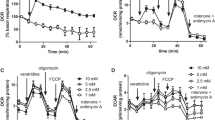
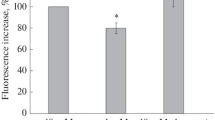
Hypoglycemia can cause neuronal cell death similar to that of glutamate-induced cell death. In the present paper, we investigated the effect of glucose removal from incubation medium on changes of mitochondrial and plasma membrane potentials in rat brain synaptosomes using the fluorescent dyes DiSC3(5) and JC-1. We also monitored pH gradients in synaptic vesicles and their recycling by the fluorescent dye acridine orange. Glucose deprivation was found to cause an inhibition of K+-induced Ca2+-dependent exocytosis and a shift of mitochondrial and plasma membrane potentials to more positive values. The sensitivity of these parameters to the energy deficit caused by the removal of glucose showed the following order: mitochondrial membrane potential > plasma membrane potential > pH gradient in synaptic vesicles. The latter was almost unaffected by deprivation compared with the control. The pH-dependent dye acridine orange was used to investigate synaptic vesicle recycling. However, the compound’s fluorescence was shown to be enhanced also by the mixture of mitochondrial toxins rotenone (10 µM) and oligomycin (5 µg/mL). This means that acridine orange can presumably be partially distributed in the intermembrane space of mitochondria. Glucose removal from the incubation medium resulted in a 3.7-fold raise of acridine orange response to rotenone + oligomycin suggesting a dramatic increase in the mitochondrial pH gradient. Our results suggest that the biophysical characteristics of neuronal presynaptic endings do not favor excessive non-controlled neurotransmitter release in case of hypoglycemia. The inhibition of exocytosis and the increase of the mitochondrial pH gradient, while preserving the vesicular pH gradient, are proposed as compensatory mechanisms.




Similar content being viewed by others
References
Isaev NK, Stel’mashuk EV, Zorov DB (2007) Cellular mechanisms of brain hypoglycemia. Biochemistry (Moscow) 72:471–478
Suh SW, Hamby AM, Swanson RA (2007) Hypoglycemia, brain energetic, and hypoglycemic neuronal death. Glia 55:1280–1286
Languren G, Montiel T, Julio-Amilpas A, Massieu L (2013) Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochem Int 63:331–343
Auer RN, Olsson Y, Siesjo BK (1984) Hypoglycemic brain injury in the rat. Correlation of density ob brain damage with the EEG isoelectric time: a quantitative study. Diabetes 33:1090–1098
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542
Wieloch T (1985) Hypoglycemia-induced neuronal damage prevented by an N-methyl-d-aspartate antagonists. Science 230:681–683
Kauppinen RA, Nicholls DG (1986) Synaptosomal bioenergetics. The role of glycolisis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem 158:159–165
Silver IA, Deas J, Erecinska M (1997) Ion homeostasis in brain cells: differences in intracellular ion responses to energy limitation between cultured neurons and glial cells. Neuroscience 78:589–601
Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA (2003) Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) inhibitors administered after hypoglycemia. J Neurosci 23:10681–10690
Kann O, Kovacs R (2007) Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292:C641–657
Chinopoulos C, Adam-Vizi V (2010) Mitochondria as ATP consumers in cellular pathology. Biochem Biophys Acta 1802:221–227
Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13:566–578
Maycox PR, Deckwerth T, Hell JW, Jahn R (1988) Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem 263:15423–15428
Zoccarato F, Cavallini L, Alexandre A (1999) The pH-sensitive dye acridine orange as a tool to monitor exocytosis/endocytosis in synaptosomes. J Neurochem 72:625–633
Wilhelm BG, Mandad S, Truckenbrodt S, Kröhnert K, Schäfer C, Rammner B, Koo SJ, Claβen GA, Krauss M, Haucke V, Urlaub H, Rizzoli SO (2014) Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344:1023–1028
Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci 15:4102–4108
Tarasenko AS, Storchak LG, Himmelreich NH (2008) Alpha-latrotoxin affects mitochondrial potential and synaptic vesicles proton gradient of nerve terminals. Neurochem Int 52:392–400
Lemeshchenko VV, Pekun TG, Vasim TV, Fedorovich SV (2012) Y-27632 induces calcium-independent glutamate release in rat brain synaptosomes by the mechanism which is distinct from exocytosis. Biofizika 57:454–459 (in Russian)
Waseem TV, Rakovich AA, Lavrukevich TV, Konev SV, Fedorovich SV (2005) Calcium regulates the mode of exocytosis induced by hypotonic shock in isolated neuronal presynaptic endings. Neurochem Int 46:235–242
Waseem TV, Kolos VA, Lapatsina LP, Fedorovich SV (2007) Hypertonic shrinking but not hypotonic swelling increases sodium concentration in rat brain synaptosomes. Brain Res Bull 73:135–142
Waseem TV, Fedorovich SV (2010) Presynaptic glycine receptors influence plasma membrane potential and glutamate release. Neurochem Res 35:1188–1195
Chinopoulos C, Tretter L, Adam-Vizi V (1999) Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of α-ketoglutarate dehydrogenase. J Neurochem 73:220–228
Hajos F (1975) An improved method for the preparation of synaptosomal fractions in high purity. Brain Res 93:485–489
Pekun TG, Lemeshchenko VV, Lyskova TI, Waseem TV, Fedorovich SV (2013) Influence of intra- and extracellular acidification on free radical formation and mitochondria membrane potential in rat brain synaptosomes. J Mol Neurosci 49:211–222
Lowry O, Rosenbrough H, Farr H, Randall R (1951) Protein measurements with Folin reagent. J Biol Chem 193:265–279
Fedorovich SV (2013) Piracetam induces plasma membrane depolarization in rat brain synaptosomes. Neurosci Lett 553:206–210
Pekun TG, Waseem TV, Fedorovich SV (2014) Depolarization of plasma membrane of rat brain synaptosomes at extra- and intracellular acidification. Biophysics 59:77–80
Blaustein MP, Goldring MP (1975) Membrane potentials in pinched-off presynaptic nerve terminals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol 247:589–615
Rosenmund C, Stevens CF (1996) Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16:1197–1207
Waseem TV, Lapatsina LP, Fedorovich SV (2008) Influence of integrin-blocking peptide on gadolinium- and hypertonic shrinking-induced neurotransmitter release in rat brain synaptosomes. Neurochem Res 33:1316–1324
Morgenthaler FD, Kraftsik R, Catsikas S, Magistretti PJ, Chatton J-Y (2006) Glucose and lactate are equally effective in energizing activity-dependent synaptic vesicle turnover in purified cortical neurons. Neuroscience 141:157–165
Lee DY, Xun Z, Platt V, Budworth H, Canaria CA, McMurray CT (2013) Distinct pools of non-glycolytic substrates differentiate brain regions and prime region-specific responses of mitochondria. Plos One 8:e68831
Südhof TC (2013) Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80:675–690
Imig C, Min SW, Krinner S, Arancillo M, Rosenmund C, Südhof TC, Rhee J, Brose N, Cooper BH (2014) The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron 84:416–431
Krisanova N, Sivko R, Kasatkina L, Borysov A, Borisova T (2014) Excitotoxic potential of exogenous ferritin and apoferritin: changes in ambient level of glutamate and synaptic vesicle acidification in brain nerve terminals. Mol Cell Neurosci 58:95–104
Van der Kloot W (2003) Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction. Prog Neurobiol 71:269–303
Melnik VI, Bikbulatova LS, Gulyaeva NV, Bazyan AS (2001) Synaptic vesicle acidification and exocytosis studied with acridine orange fluorescence in rat brain synaptosomes. Neurochem Res 26:549–554
Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, Rugolo M (2005) pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Biophys Res Commun 326:799–804
Wolosker H, de Souza DO, de Meis L (1996) Regulation of glutamate transport into synaptic vesicles by chloride and proton gradient. J Biol Chem 271:11726–11731
Tretter L, Chinopoulos C, Adam-Vizi V (1998) Plasma membrane depolarization and disturbed Na+ homeostasis induced by the protonophore carbonyl cyanide-p-trifluoromethoxyphenyl-hydrazon in isolated nerve terminals. Mol Pharmacol 53:734–741
Alekseenko AV, Lemeshchenko VV, Pekun TG, Waseem TV, Fedorovich SV (2012) Glutamate-induced free radical formation in rat brain synaptosomes is not dependent on intrasynaptosomal mitochondria membrane potential. Neurosci Lett 513:238–242
Patten DA, Wong J, Khacho M, Soubanner V, Mailloux RJ, Pilon-Larose K, MacLaurin JG, Park DS, McBride HM, Trinkle-Mulcahy L, Harper M-E, Germain M, Slack RS (2014) OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J 33:2676–2691
Edwards RH (2007) The neurotransmitter cycle and quantal size. Neuron 55:835–858
Hell JW, Maycox PR, Jahn R (1990) Energy dependence and functional reconstitution of the γ-aminobutyric acid carrier from synaptic vesicles. J Biol Chem 265:2111–2117
Acknowledgments
This work was supported by Belorussian Republican Foundation of Basic Investigation (Grant B13-066). Foundation body had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hrynevich, S.V., Pekun, T.G., Waseem, T.V. et al. Influence of Glucose Deprivation on Membrane Potentials of Plasma Membranes, Mitochondria and Synaptic Vesicles in Rat Brain Synaptosomes. Neurochem Res 40, 1188–1196 (2015). https://doi.org/10.1007/s11064-015-1579-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1579-0