Abstract
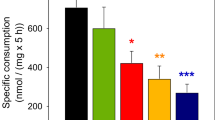
It is well established that astrocytes can utilize many substrates to support oxidative energy metabolism; however, use of energy substrates in the presence of other substrates, as would occur in vivo, has not been systematically evaluated. Substrate competition studies were used to determine changes in the rates of 14CO2 production since little is known about the interaction of energy substrates in astrocytes. The rates of 14CO2 production from 1 mM D-[6-14C]glucose, l-[U-14C]glutamate, l-[U-14C]glutamine, d-3-hydroxy[3-14C]butyrate, l-[U-14C]lactate and l-[U-14C]malate by primary cultures of astrocytes from rat brain were determined to be 1.17 ± 0.19, 85.30 ± 12.25, 28.04 ± 2.84, 13.55 ± 4.56, 14.84 ± 2.40 and 5.20 ± 1.20 nmol/h/mg protein (mean ± SEM), respectively. The rate of 14CO2 production from glutamate oxidation was higher than that of the other substrates Addition of unlabeled glutamate significantly decreased the rates of 14CO2 production from all other substrates studied; however, glutamate oxidation was not altered by the addition of any of the other substrates. The rate of 14CO2 production of glutamine was decreased by glutamate, but not altered by other substrates. The rate of 14CO2 production from glucose was significantly decreased by the addition of unlabeled glutamate, glutamine or lactate, but not by 3-hydroxybutyrate or malate. Addition of unlabeled glucose did not significantly alter the 14CO2 production from any other substrate. 14CO2 production from lactate was decreased by the addition of unlabeled glutamine or glutamate and increased by addition of malate. The 14CO2 production from malate was decreased by the addition of unlabeled glutamate or lactate, but was not altered by the other substrates. The substrate utilization for oxidative energy metabolism in astrocytes is very different than the profile previously reported for synaptic terminals. These studies demonstrate the potential use of multiple substrates including glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate as energy substrates for astrocytes. The data also provide evidence of interactions of substrates and multiple compartments of TCA cycle activity in cultured astrocytes.


Similar content being viewed by others
References
Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M (2007) The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci 27:12255–12266
Hertz L (2006) Glutamate, a neurotransmitter—and so much more. A synopsis of Wierzba III. Neurochem Int 48:416–425
Gruetter R, Adriany G, Choi IY, Henry PG, Lei H, Oz G (2003) Localized in vivo 13C NMR spectroscopy of the brain. NMR Biomed 16:313–338
Bluml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD (2002) Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed 15:1–5
Hertz L, Peng L, Dienel GA (2007) Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27:219–249
Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A (2001) Multiple compartments with different metabolic characteristics are involved in biosynthesis of intracellular and released glutamine and citrate in astrocytes. Glia 35:246–252
Qu H, Eloqayli H, Unsgard G, Sonnewald U (2001) Glutamate decreases pyruvate carboxylase activity and spares glucose as energy substrate in cultured cerebellar astrocytes. J Neurosci Res 66:1127–1132
Peng L, Swanson RA, Hertz L (2001) Effects of l-glutamate, d-aspartate, and monensin on glycolytic and oxidative glucose metabolism in mouse astrocyte cultures: further evidence that glutamate uptake is metabolically driven by oxidative metabolism. Neurochem Int 38:437–443
McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S (1993) Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci 15:320–329
McKenna MC, Tildon JT, Couto R, Stevenson JH, Caprio FJ (1990) The metabolism of malate by cultured rat brain astrocytes. Neurochem Res 15:1211–1220
McKenna MC, Hopkins IB, Carey A (2001) Alpha-cyano-4-hydroxycinnamate decreases both glucose and lactate metabolism in neurons and astrocytes: implications for lactate as an energy substrate for neurons. J Neurosci Res 66:747–754
Hertz L, Hertz E (2003) Cataplerotic TCA cycle flux determined as glutamate-sustained oxygen consumption in primary cultures of astrocytes. Neurochem Int 43:355–361
Hertz L, Drejer J, Schousboe A (1988) Energy metabolism in glutamatergic neurons, GABAergic neurons and astrocytes in primary cultures. Neurochem Res 13:605–610
Hertz L (2003) Astrocytic amino acid metabolism under control conditions and during oxygen and/or glucose deprivation. Neurochem Res 28:243–258
Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J (1987) Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 18:551–561
Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A (1998) Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev Neurosci 20:310–320
Sonnewald U, Westergaard N, Schousboe A (1997) Glutamate transport and metabolism in astrocytes. Glia 21:56–63
Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Huang R, Peng L, Hertz L (1993) Glutamate and glutamine metabolism and compartmentation in astrocytes. Dev Neurosci 15:359–366
Dienel GA (2012) Fueling and imaging brain activation. ASN Neuro 4(5):e00093. doi:10.1042/AN20120021
McKenna MC, Dienel GA, Waagepetersen HS, Schousboe A, Sonnewald U (2011) Energy metabolism of the brain. In: Brady S, Price D, Siegal G (eds) Basic neurochemistry, vol 8. Blackwell, Oxford
Lai JC, Murthy CR, Cooper AJ, Hertz E, Hertz L (1989) Differential effects of ammonia and beta-methylene-DL-aspartate on metabolism of glutamate and related amino acids by astrocytes and neurons in primary culture. Neurochem Res 14:377–389
Yudkoff M, Nissim I, Pleasure D (1988) Astrocyte metabolism of [15N]glutamine: implications for the glutamine-glutamate cycle. J Neurochem 51:843–850
Yu AC, Fisher TE, Hertz E, Tildon JT, Schousboe A, Hertz L (1984) Metabolic fate of [14C]-glutamine in mouse cerebral neurons in primary cultures. J Neurosci Res 11:351–357
Zielke HR, Tildon JT, Landry ME, Max SR (1990) Effect of 8-bromo-cAMP and dexamethasone on glutamate metabolism in rat astrocytes. Neurochem Res 15:1115–1122
McKenna MC, Tildon JT, Stevenson JH, Huang X (1996) New insights into the compartmentation of glutamate and glutamine in cultured rat brain astrocytes. Dev Neurosci 18:380–390
McKenna MC (2003) Glutamate metabolism in primary cultures of rat brain astrocytes: rationale and initial efforts toward developing a compartmental model. Adv Exp Med Biol 537:317–341
Edmond J, Auestad N, Robbins RA, Bergstrom JD (1985) Ketone body metabolism in the neonate: development and the effect of diet. Fed Proc 44:2359–2364
Tildon JT, McKenna MC, Stevenson JH Jr (1994) Transport of 3-hydroxybutyrate by cultured rat brain astrocytes. Neurochem Res 19:1237–1242
Lopes-Cardozo M, Larsson OM, Schousboe A (1986) Acetoacetate and glucose as lipid precursors and energy substrates in primary cultures of astrocytes and neurons from mouse cerebral cortex. J Neurochem 46:773–778
Dienel GA, Cruz NF (2003) Neighborly interactions of metabolically-activated astrocytes in vivo. Neurochem Int 43:339–354
Tildon JT, McKenna MC, Stevenson J, Couto R (1993) Transport of l-lactate by cultured rat brain astrocytes. Neurochem Res 18:177–184
Sickmann HM, Schousboe A, Fosgerau K, Waagepetersen HS (2005) Compartmentation of lactate originating from glycogen and glucose in cultured astrocytes. Neurochem Res 30:1295–1304
Qu H, Eloqayli H, Sonnewald U (2005) Pentylenetetrazole affects metabolism of astrocytes in culture. J Neurosci Res 79:48–54
Oh YJ, Markelonis GJ, Oh TH (1991) Immunocytochemical localization of mitochondrial malate dehydrogenase in primary cultures of rat astrocytes and oligodendrocytes. J Histochem Cytochem 39:681–688
Cesar M, Hamprecht B (1995) Immunocytochemical examination of neural rat and mouse primary cultures using monoclonal antibodies raised against pyruvate carboxylase. J Neurochem 64:2312–2318
Cremer JE (1981) Nutrients for the brain: problems in supply. Early Hum Dev 5:117–132
Cremer JE (1982) Substrate utilization and brain development. J Cereb Blood Flow Metab 2:394–407
Vannucci SJ, Simpson IA (2003) Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab 285:E1127–E1134
Yu AC, Drejer J, Hertz L, Schousboe A (1983) Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem 41:1484–1487
Lapidot A, Gopher A (1994) Cerebral metabolic compartmentation. Estimation of glucose flux via pyruvate carboxylase/pyruvate dehydrogenase by 13C NMR isotopomer analysis of d-[U-13C]glucose metabolites. J Biol Chem 269:27198–27208
Qu H, Haberg A, Haraldseth O, Unsgard G, Sonnewald U (2000) (13)C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci 22:429–436
Sonnewald U, Westergaard N, Hassel B, Muller TB, Unsgard G, Fonnum F, Hertz L, Schousboe A, Petersen SB (1993) NMR spectroscopic studies of 13C acetate and 13C glucose metabolism in neocortical astrocytes: evidence for mitochondrial heterogeneity. Dev Neurosci 15:351–358
Peng L, Zhang X, Hertz L (1994) High extracellular potassium concentrations stimulate oxidative metabolism in a glutamatergic neuronal culture and glycolysis in cultured astrocytes but have no stimulatory effect in a GABAergic neuronal culture. Brain Res 663:168–172
Dienel GA, Cruz NF (2009) Exchange-mediated dilution of brain lactate specific activity: implications for the origin of glutamate dilution and the contributions of glutamine dilution and other pathways. J Neurochem 109(Suppl 1):30–37
Cerdan S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, Garcia-Martin ML (2006) The redox switch/redox coupling hypothesis. Neurochem Int 48:523–530
Brown AM, Ransom BR (2007) Astrocyte glycogen and brain energy metabolism. Glia 55:1263–1271
Alves PM, McKenna MC, Sonnewald U (1995) Lactate metabolism in mouse brain astrocytes studied by [13C] NMR spectroscopy. NeuroReport 6:2201–2204
Abe T, Takahashi S, Suzuki N (2006) Oxidative metabolism in cultured rat astroglia: effects of reducing the glucose concentration in the culture medium and of d-aspartate or potassium stimulation. J Cereb Blood Flow Metab 26:153–160
Hertz L, Dienel GA (2005) Lactate transport and transporters: general principles and functional roles in brain cells. J Neurosci Res 79:11–18
Gandhi GK, Cruz NF, Ball KK, Theus SA, Dienel GA (2009) Selective astrocytic gap junctional trafficking of molecules involved in the glycolytic pathway: impact on cellular brain imaging. J Neurochem 110:857–869
Zielke HR, Zielke CL, Baab PJ, Tildon JT (2007) Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem 101:9–16
McKenna MC, Sonnewald U (2005) GABA alters the metabolic fate of [U-13C]glutamate in cultured cortical astrocytes. J Neurosci Res 79:81–87
McKenna MC, Sonnewald U, Huang X, Stevenson J, Johnsen SF, Sande LM, Zielke HR (1998) Alpha-ketoisocaproate alters the production of both lactate and aspartate from [U-13C]glutamate in astrocytes: a 13C NMR study. J Neurochem 70:1001–1008
McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66:386–393
Schousboe A, Westergaard N, Waagepetersen HS, Larsson OM, Bakken IJ, Sonnewald U (1997) Trafficking between glia and neurons of TCA cycle intermediates and related metabolites. Glia 21:99–105
Qu H, Konradsen JR, van Hengel M, Wolt S, Sonnewald U (2001) Effect of glutamine and GABA on [U-(13)C]glutamate metabolism in cerebellar astrocytes and granule neurons. J Neurosci Res 66:885–890
Westergaard N, Sonnewald U, Schousboe A (1995) Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Dev Neurosci 17:203–211
Sonnewald U, Westergaard N, Petersen SB, Unsgard G, Schousboe A (1993) Metabolism of [U-13C]glutamate in astrocytes studied by 13C NMR spectroscopy: incorporation of more label into lactate than into glutamine demonstrates the importance of the tricarboxylic acid cycle. J Neurochem 61:1179–1182
Schousboe A, Waagepetersen HS (2005) Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox Res 8:221–225
Schousboe A, Svenneby G, Hertz L (1977) Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem 29:999–1005
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J (1995) Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15:711–720
Kreft M, Bak LK, Waagepetersen HS, Schousboe A (2012) Aspects of astrocyte energy metabolism, amino acid neurotransmitter homeostasis and metabolic compartmentation. ASN Neuro 4(3):e00086. doi:10.1042/AN20120007
Westergaard N, Drejer J, Schousboe A, Sonnewald U (1996) Evaluation of the importance of transamination versus deamination in astrocytic metabolism of [U-13C]glutamate. Glia 17:160–168
Malik P, McKenna MC, Tildon JT (1993) Regulation of malate dehydrogenases from neonatal, adolescent, and mature rat brain. Neurochem Res 18:247–257
McKenna MC, Tildon JT, Stevenson JH, Hopkins IB (1994) Energy metabolism in cortical synaptic terminals from weanling and mature rat brain: evidence for multiple compartments of tricarboxylic acid cycle activity. Dev Neurosci 16:291–300
McKenna MC, Bezold LI, Kimatian SJ, Tildon JT (1986) Competition of glycerol with other oxidizable substrates in rat brain. Biochem J 237:47–51
McKenna MC, Hopkins IB, Lindauer SL, Bamford P (2006) Aspartate aminotransferase in synaptic and nonsynaptic mitochondria: differential effect of compounds that influence transient hetero-enzyme complex (metabolon) formation. Neurochem Int 48:629–636
McKenna MC, Tildon JT, Stevenson JH, Huang X, Kingwell KG (1995) Regulation of mitochondrial and cytosolic malic enzymes from cultured rat brain astrocytes. Neurochem Res 20:1491–1501
Hertz L, Juurlink BHJ, Fosmark H, Schousboe A (eds) (1982) Astrocytes in primary culture. In: Pfeiffer SE (ed) Neuroscience approached through cell culture, Vol 1. CRC Press, Boca Raton, pp 175–186
Chechik T, Roeder LM, Tildon JT, Poduslo SE (1987) Ketone body enzyme activities in purified neurons, astrocytes and oligodendroglia. Neurochem Int 10:95–99
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Schousboe A, Sickmann HM, Bak LK, Schousboe I, Jajo FS, Faek SA, Waagepetersen HS (2011) Neuron-glia interactions in glutamatergic neurotransmission: roles of oxidative and glycolytic adenosine triphosphate as energy source. J Neurosci Res 89:1926–1934
Li B, Hertz L, Peng L (2012) Aralar mRNA and protein levels in neurons and astrocytes freshly isolated from young and adult mouse brain and in maturing cultured astrocytes. Neurochem Int. doi:10.1016/j.neuint.2012.09.009
Berkich DA, Ola MS, Cole J, Sweatt AJ, Hutson SM, LaNoue KF (2007) Mitochondrial transport proteins of the brain. J Neurosci Res 85:3367–3377
Bergles DE, Diamond JS, Jahr CE (1999) Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol 9:293–298
McKenna MC (2007) The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res 85:3347–3358
Gruetter R, Seaquist ER, Ugurbil K (2001) A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab 281:E100–E112
Edmond J (1992) Energy metabolism in developing brain cells. Can J Physiol Pharmacol 70(Suppl):S118–S129
Schousboe A, Sonnewald U, Civenni G, Gegelashvili G (1997) Role of astrocytes in glutamate homeostasis. Implications for excitotoxicity. Adv Exp Med Biol 429:195–206
Yudkoff M, Nissim I, Daikhin Y, Lin ZP, Nelson D, Pleasure D, Erecinska M (1993) Brain glutamate metabolism: neuronal-astroglial relationships. Dev Neurosci 15:343–350
Yudkoff M, Nissim I, Hummeler K, Medow M, Pleasure D (1986) Utilization of [15N]glutamate by cultured astrocytes. Biochem J 234:185–192
Pellerin L, Magistretti PJ (1996) Excitatory amino acids stimulate aerobic glycolysis in astrocytes via an activation of the Na+/K+ ATPase. Dev Neurosci 18:336–342
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Dienel GA (2012) Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 32:1107–1138
Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O’Donnell JC, Spruce LA, Xiao R, Guo W, Putt M, Seeholzer S, Ischiropoulos H, Robinson MB (2011) Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci 31:18275–18288
Waagepetersen HS, Qu H, Hertz L, Sonnewald U, Schousboe A (2002) Demonstration of pyruvate recycling in primary cultures of neocortical astrocytes but not in neurons. Neurochem Res 27:1431–1437
Olstad E, Olsen GM, Qu H, Sonnewald U (2007) Pyruvate recycling in cultured neurons from cerebellum. J Neurosci Res 85:3318–3325
Sonnewald U, Westergaard N, Jones P, Taylor A, Bachelard HS, Schousboe A (1996) Metabolism of [U-13C5]glutamine in cultured astrocytes studied by NMR spectroscopy: first evidence of astrocytic pyruvate recycling. J Neurochem 67:2566–2572
Waagepetersen HS, Sonnewald U, Qu H, Schousboe A (1999) Mitochondrial compartmentation at the cellular level: astrocytes and neurons. Ann N Y Acad Sci 893:421–425
Hertz L (2004) Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem Int 45:285–296
Bakken IJ, White LR, Aasly J, Unsgard G, Sonnewald U (1997) Lactate formation from [U-13C]aspartate in cultured astrocytes: compartmentation of pyruvate metabolism. Neurosci Lett 237:117–120
Takahashi S, Izawa Y, Suzuki N (2012) Astroglial pentose phosphate pathway rates in response to high-glucose environments. ASN Neuro 4:71–88
Bolanos JP, Cidad P, Garcia-Nogales P, Delgado-Esteban M, Fernandez E, Almeida A (2004) Regulation of glucose metabolism by nitrosative stress in neural cells. Mol Aspects Med 25:61–73
Garcia-Nogales P, Almeida A, Fernandez E, Medina JM, Bolanos JP (1999) Induction of glucose-6-phosphate dehydrogenase by lipopolysaccharide contributes to preventing nitric oxide-mediated glutathione depletion in cultured rat astrocytes. J Neurochem 72:1750–1758
Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP (2009) The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol 11:747–752
Halim ND, McFate T, Mohyeldin A, Okagaki P, Korotchkina LG, Patel MS, Jeoung NH, Harris RA, Schell MJ, Verma A (2010) Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia 58:1168–1176
McKenna MC, Tildon JT, Stevenson JH, Hopkins IB, Huang X, Couto R (1998) Lactate transport by cortical synaptosomes from adult rat brain: characterization of kinetics and inhibitor specificity. Dev Neurosci 20:300–309
Pellerin L, Magistretti PJ (2011) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32:1152–1166
Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262
Barros LF, Deitmer JW (2010) Glucose and lactate supply to the synapse. Brain Res Rev 63:149–159
Pellerin L (2005) How astrocytes feed hungry neurons. Mol Neurobiol 32:59–72
DiNuzzo M, Mangia S, Maraviglia B, Giove F (2010) Changes in glucose uptake rather than lactate shuttle take center stage in subserving neuroenergetics: evidence from mathematical modeling. J Cereb Blood Flow Metab 30:586–602
Mangia S, Simpson IA, Vannucci SJ, Carruthers A (2009) The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem 109(Suppl 1):55–62
DiNuzzo M, Mangia S, Maraviglia B, Giove F (2010) Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J Cereb Blood Flow Metab 30:1895–1904
Zhang F, Vannucci SJ, Philp NJ, Simpson IA (2005) Monocarboxylate transporter expression in the spontaneous hypertensive rat: effect of stroke. J Neurosci Res 79:139–145
Nehlig A (2004) Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fatty Acids 70:265–275
Nehlig A (1999) Age-dependent pathways of brain energy metabolism: the suckling rat, a natural model of the ketogenic diet. Epilepsy Res 37:211–221
Pan JW, de Graaf RA, Petersen KF, Shulman GI, Hetherington HP, Rothman DL (2002) [2,4-13 C2]-beta-Hydroxybutyrate metabolism in human brain. J Cereb Blood Flow Metab 22:890–898
Waagepetersen HS, Qu H, Schousboe A, Sonnewald U (2001) Elucidation of the quantitative significance of pyruvate carboxylation in cultured cerebellar neurons and astrocytes. J Neurosci Res 66:763–770
Huang Y, Zielke CL, Tildon JT, Zielke HR (1993) Monitoring in vivo oxidation of 14C-labelled substrates to 14CO2 by brain microdialysis. Dev Neurosci 15:233–239
Duffy TE, Nelson SR, Lowry OH (1972) Cerebral carbohydrate metabolism during acute hypoxia and recovery. J Neurochem 19:959–977
Hindfelt B, Plum F, Duffy TE (1977) Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J Clin Invest 59:386–396
Williamson DH, Lund P, Krebs HA (1967) The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103:514–527
Acknowledgments
This work was supported in part by NIH grant HD16596. The technical assistance of Irene Hopkins and Joseph Stevenson is gratefully acknowledged for performing these studies. I thank Dr. Leif Hertz for his insightful comments and feedback regarding this manuscript and his pioneering work in the field of brain energy metabolism.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In Honor of Leif Hertz.
Rights and permissions
About this article
Cite this article
McKenna, M.C. Substrate Competition Studies Demonstrate Oxidative Metabolism of Glucose, Glutamate, Glutamine, Lactate and 3-Hydroxybutyrate in Cortical Astrocytes from Rat Brain. Neurochem Res 37, 2613–2626 (2012). https://doi.org/10.1007/s11064-012-0901-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0901-3