Abstract
Despite aggressive management consisting of surgery, radiation therapy (RT), and systemic therapy given alone or in combination, a significant proportion of patients with brain tumors will experience tumor recurrence. For these patients, no standard of care exists and management of either primary or metastatic recurrent tumors remains challenging.
Advances in imaging and RT technology have enabled more precise tumor localization and dose delivery, leading to a reduction in the volume of health brain tissue exposed to high radiation doses. Radiation techniques have evolved from three-dimensional (3-D) conformal RT to the development of sophisticated techniques, including intensity modulated radiation therapy (IMRT), volumetric arc therapy (VMAT), and stereotactic techniques, either stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT). Several studies have suggested that a second course of RT is a feasible treatment option in patients with a recurrent tumor; however, survival benefit and treatment related toxicity of reirradiation, given alone or in combination with other focal or systemic therapies, remain a controversial issue.
We provide a critical overview of the current clinical status and technical challenges of reirradiation in patients with both recurrent primary brain tumors, such as gliomas, ependymomas, medulloblastomas, and meningiomas, and brain metastases. Relevant clinical questions such as the appropriate radiation technique and patient selection, the optimal radiation dose and fractionation, tolerance of the brain to a second course of RT, and the risk of adverse radiation effects have been critically discussed.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation therapy (RT) remains an integral part of the management of most tumors of the central nervous system (CNS) [1]. Techniques have significantly improved in the last years resulting in new approaches to deliver highly conformal RT to the tumor while limiting the radiation dose to surrounding health brain tissues and organs at risk (OARs) [2]. Stereotactic radiotherapy (SRT), given as single-fraction stereotactic radiosurgery (SRS) or fractionated SRT, is frequently used for the treatment of small to medium size lesions due to its superiority in terms of dose conformity and rapid dose fall off outside the target compared with three-dimensional (3-D) conformal RT. Advanced techniques such as intensity-modulated RT (IMRT) and volumetric modulated arc therapy (VMAT) optimize the delivery of irradiation to large and irregularly shaped volumes. Finally, the use of appropriate image guided RT (IGRT) systems, using either orthogonal x-rays or cone beam computed tomography (CBCT), is necessary to reduce set-up margins and achieve high-precise patient positioning during treatment. Currently, there is a renewed interest in particle therapy using protons or heavier ions, because of their intrinsic physical and biological properties that consent to maintain highly conformal target coverage while sparing normal surrounding tissues and reducing the integral dose to the patient compared to most modern photon techniques [3].
In case of tumor relapse or progression, treatment options include surgery, chemotherapy or reirradiation, alone or in combination, as potentially salvage strategies. Due to its complexity, all treatment decisions require a multidisciplinary approach and should consider patients specific characteristics. Historically, radiation oncologists have been cautious regarding a second course of RT for tumors that have recurred in or close to the original treatment volume because of concerns about the risks of adverse radiation effects, such as radiation-induced brain necrosis. However, evidence from preclinical animal models and results from clinical series shows that brain and spinal cord have marked repair potential suggesting that reirradiation may represent a feasible option in selected patients [4,5,6,7,8]. When performing reirradiation, it is essential to keep dose as low as possible to normal brain tissue surrounding the recurrent tumor and sensitive structures such as brain stem, spinal cord, and optic apparatus. With this purpose, high-precision stereotactic techniques that allow for highly accurate patient positioning and dose delivery have replaced conventional RT in clinical practice for the treatment of patients with recurrent tumors who are deemed to receive reirradiation [2].
We performed an overview of the available literature on reirradiation of different types of primary brain tumors, such as gliomas, ependymomas, medulloblastomas and meningiomas, and brain metastases. Radiobiological principles behind reirradiation and current clinical evidence on the efficacy and toxicity of reirradiation have been critically discussed.
Methods
We conducted a literature search of the relevant data on reirradiation of brain tumors using PubMed and Scopus databases, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The following combination of keywords were searched: “reirradiation”, “recurrent brain tumors”, “glioblastoma”, “ependymoma”, “medulloblastoma”, “brain metastases”, “diffuse intrinsic pontine glioma” and “meningioma”. Search was limited to papers published in English language from January 1997 to February 2023. Clinical trials, original research, review articles, case report and case series were included, and reference lists were carefully explored for relevant papers that would have been missed by electronic search. Based on the initial searches, a total of 732 articles were identified. After abstract screening, 593 articles were excluded (duplicates or irrelevant) and 139 articles were considered for full text review. Studies assessing the role of RT in recurrent CNS tumors but without details on clinical outcomes and/or reirradiation were excluded (n = 70). A total number of 69 papers were finally reviewed based on relevance to the scope of this review. Data on overall survival (OS), progression-free survival (PFS), and toxicity after photon or particle reirradiation were extracted and grouped according to the clinical scenario investigated.
Brain tolerance to reirradiation and dose constraints
Normal brain tissue dose tolerance is the limiting factor when giving reirradiation. Data on tolerance and recovery of CNS structures come from both preclinical and clinical studies [4,5,6,7,8]. Experimental data from studies investigating spinal cord tolerance to irradiation indicate that the CNS has a capacity to recover from occult radiation injury following treatment [4, 5]. In pigs treated with prior RT at a total dose of 30 Gy given in 10 fractions of 3 Gy each, Medin et al. [5] showed that spinal SRS reirradiation performed after one year was not significantly associated with an increased risk of motor deficits compared to controls treated by SRS only [5]. In another series of 56 rhesus monkeys assigned to receive two radiation courses to the cervical and upper thoracic spinal cord, 44 Gy during the first course and 57.2 Gy at the time of reirradiation given in 2.2 Gy per fraction, Ang et al. [4] reported a substantial recovery of occult injury within the first year following the initial course of RT. An additional recovery up to 100% was seen between 1 and 3 years, with no evidence of myelopathy for cumulative dose less than 110 Gy. Based on these experimental data, the authors suggested to use an estimated recovery of occult injury after a first course of RT around 50% at 1 year, 60% at 2 years, and 65–70% at 3 years or more, for the reirradiation of selected patients. Overall, for reirradiation of the full cord cross-section at 2 Gy per day after prior conventionally fractionated treatment, cord tolerance appears to increase at least 25% six months after the initial course of RT [6,7,8]. Although most data refer to spinal cord tolerance, the pathogenesis of brain radiation toxicity and its potential recovery is assumed to be like the one in spinal cord because of their low α/β ratio.
An estimated risk of symptomatic brain necrosis has been determined in patients with brain tumors following both SRS and SRT [6, 9,10,11]. A risk around 5% can be predicted following biologically equivalent dose (BED) of 72 Gy (range, 60–84 Gy) and 90 Gy (range, 84–102 Gy) with standard fractionation (1.8 to 2.0 Gy per fraction). For single-fraction SRS, volumes of normal brain receiving 12 Gy (V12Gy) of 5 cc, 10 cc, or > 15 cc have been associated with a risk of symptomatic radionecrosis around 10%, 15%, and 20%, respectively [10, 11]. Thus, the quantitative analysis of normal tissue effects in the clinic (QUANTEC) recommends limiting single-fraction V12Gy to ≤ 5–10 cc (7). Based on dose/volume data and clinical risk estimates, maximum doses exceeding 55 Gy and 54 Gy in 1.8-2 Gy fractions for optic apparatus and brainstem, respectively, should be avoided in current clinical practice [12,13,14]. Following SRS, a maximum dose of 12 Gy to brainstem and 8 Gy to the optic apparatus is recommended [12, 14, 15]; of note, the risk of optic neuropathy remains low for maximum point doses of 10 to 12 Gy to small portions of the optic apparatus [16,17,18,19]. Data on tolerance doses of CNS organs at risk (OARs) to fractionated RT (3 to 5 fractions) are relatively limited and dose constraints remain not validated [20, 21]. Current clinical recommendations indicate that doses to brainstem, optic apparatus, and spine should not exceed a maximum point dose of 23 Gy, 19.5 Gy, and 22.5 Gy when using 3 fractions, and 31 Gy, 25 Gy, and 30 Gy when using 5 fractions, respectively.
Different factors may alter the risk of radionecrosis following reirradiation for brain tumors, including dose and fractionation, target volumes, combined systemic treatments, and interval between the RT treatment courses [22, 23]. In their meta-analysis of 30 studies published from 1996 to 2011, Sminia and Mayer [22] found no cases of radionecrosis for a cumulative biologically effective dose normalized to 2 Gy/fraction (EQD2) of < 96 Gy using the linear quadratic model and assuming an α/β ratio of 2 Gy for normal health brain. The median cumulative EQD2 was generally higher in SRS series (111.6 to 137.2 Gy) than in hypofractionated (90 to 133.9 Gy) and conventionally fractionated reirradiation (81.6 to 101.9 Gy) series. For patients with recurrent glioblastoma receiving hypofractionated SRT or SRS, brain necrosis was reported in 2–12% for a cumulative EQD2 > 96.2 Gy and up to 17% of for a cumulative EQD2 > 137 Gy. In recent update of the literature on reirradiation of glioblastoma, a similar risk of radionecrosis of 0 to 3% has been shown after conventional fractionation with cumulative EQD2 doses < 101 Gy, of 7 to 13% after hypofractionated SRT with cumulative EQD2 doses of 102 to 130 Gy, and up to 24.4% after SRS with cumulative EQD2 doses of 120 to 150 Gy [23].
The relationship between cumulative EQD2 values and risk of toxicity for sensitive brain structures has been evaluated in few retrospective studies [24, 25]. Niyazi et al. [24] found no relevant long-term toxicity in a series of 58 patients who received reirradiation for a malignant glioma using maximum EQD2 values of 80.3 Gy, 79.4 and 95.2 Gy to the optic chiasm, optic nerves and brainstem, respectively, considering an α/β ratio of 2 Gy for these structures. In a systematic review on reirradiation of diffuse brainstem gliomas including seven studies with a total of 90 patients, Lu et al. [25] showed that a second course of radiation was associated with clinical improvement and radiological response without significant toxicity employing doses of 20–24 Gy given in 2 Gy fractions. Overall, these data indicate relatively high and fast recovery capacity of the normal human brain after RT like those seen for spine [4, 5], and support the relative safety of reirradiation using cumulative EQD2 doses around 100 Gy, or even higher (up to 120 Gy) for small and well-defined recurrent tumors away from eloquent areas.
Radiotherapy techniques and target delineation
High accuracy in tumor localization, target dose coverage and dose delivery are crucial when performing a second course of focal RT. In this setting, stereotactic techniques are frequently employed for their ability to achieve a steep dose fall-off at the edge of the target volume lowering the radiation dose to surrounding sensitive brain structures. Current stereotactic techniques include Gamma Knife (Elekta Instruments AB, Stockholm, Sweden) and linear accelerator (LINAC)-based SRS systems, such as CyberKnife (Accuray, Sunnyvale, CA, USA) or Novalis (NTx) (BrainLAB AG, Feldkirchen, Germany). Patients receiving Gamma Knife SRS are traditionally placed in a rigid stereotactic frame with a submillimetric target accuracy, while those treated with LINAC-based frameless SRS systems are usually immobilized using a thermoplastic mask. A submillimeter accuracy of patient positioning is achieved using modern image guided radiation therapy (IGRT) technologies, such as orthogonal x-rays (ExacTrac®Xray 6D system) or cone beam CT (CBCT) [2]. Radiation dose is usually delivered in a single fraction to targets smaller than 3 cm in size, while hypofractionated and conventionally fractionated schedules are frequently used for treating larger recurrent tumors. Highly conformal dose distribution can be achieved with IMRT and VMAT techniques. Currently, no comparative studies have demonstrated the clinical superiority of a technique over another in patients with brain tumors in terms of local control and treatment-related toxicity. In proton therapy, there are two main techniques of irradiation, namely active scanning or pencil beam scanning and passive scattering proton therapy. Limited data suggest that proton therapy is an effective treatment for recurrent brain tumors [26, 27], although there are no controlled studies demonstrating its superiority in comparison to photon RT in terms of local control and decreased toxicity.
An accurate delineation of tumor volumes and OARs is essential for a precise calculation of the spatial dose distribution and for the optimal radiation schedule. For brain tumors, the gross tumor volume (GTV) is generally defined as the visible lesion on MRI contrast-enhanced T1-weighted sequences. The clinical target volume (CTV), which includes areas of potential suspected microscopic tumor infiltration and potential paths of microscopic spread, can be generated by adding a variable margin of up to 5 mm to the GTV constrained at anatomical borders, e.g. tentorium, falx cerebri, and bone. In general, little (1–2 mm) or no GTV-to-CTV margins are used during SRS with the aim to limit the risk of toxicity, where larger margins up to 5 mm are commonly applied during hypofractionated and conventionally fractionated SRT [23]. Advanced MRI techniques, e.g. diffusion MRI and perfusion MRI, and positron emission tomotherapy (PET)/CT imaging with radiolabeled amino acids may help to improve target volume delineation accuracy by revealing tumor infiltration, although their use in clinical practice are limited and more evidence need to confirm their usefulness [28]. Finally, depending on radiation technique and available technology, an expansion of 0 to 3 mm is applied to generate the planning target volume (PTV) which accounts for uncertainties in treatment planning and patient positioning. Whole brain radiation therapy (WBRT) and craniospinal irradiation (CSI) can be used for selected patients with recurrent tumors that have spread into the brain and spinal cord through the cerebrospinal fluid, e.g. ependymoma and medulloblastoma.
Glioblastoma
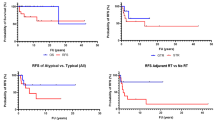
Reirradiation is increasingly used as treatment option in patients with recurrent glioblastoma after standard chemoradiation [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59]. A summary of selected reirradiation studies reporting survival and toxicity rates after different radiation schedules with or without systemic therapies is shown in Table 1. Median survival times from 7 to 13 months and 1-year OS rates of 30–55% have been observed following either SRS or fractionated SRT, with 1-year incidence of neurological toxicities ranging from 5 to 20%. Minniti et al. [23] reported the clinical outcomes of 901 patients treated with single-fraction SRS for recurrent glioblastoma included in 16 studies published between 2005 and 2020. Using a median dose of 15–18 Gy, median OS ranged from 7.5 to 13 months and median PFS from 4.4 to 6 months. Even though Gamma Knife was the most used SRS technique, clinical results were no different for patients treated with Cyberknife or LINAC-based SRS. In a recent systematic review of reirradiation with different SRS modalities for recurrent glioblastoma including 50 studies (2096 patients), Kazmi et al. [60] observed similar 12-month OS and PFS rates of 34% and 16%, respectively.
Hypofractionated SRT, given as moderate hypofractionation (35-37.5 Gy in 10–15 fractions of 2.5–3.5 Gy each) or as high-dose hypofractionation (27–35 Gy in 3–5 fractions of 5–9 Gy each) is increasingly used in the setting of reirradiation as an alternative to single-fraction SRS (Table 1) [31, 34,35,36,37,38,39, 43, 50, 53,54,55, 59]. Fogh et al. [36] observed a median OS of 11 months in 105 patients with relapsed glioblastoma who received a total dose of 35 Gy in 10 fractions. In a recent review reporting the outcome of hypofractionated SRT for 995 patients with recurrent glioblastoma included in 17 studies, a similar median OS time of 9.2 months (ranging from 7.5 to 12.5 months) has been observed in patients treated with SRT using doses of 30–45 Gy in 2.5-4.0 Gy fractions and those receiving 25–35 Gy in 5–7 Gy fractions [23].
A similar OS of 7 to10 months has been observed using conventionally fractionated SRT in 2 Gy fractions [30, 41, 51, 58]. In 172 patients with recurrent low- and high-grade gliomas who were treated with 36 Gy delivered in 2-Gy fractions, Combs et al. [30] observed median OS and PFS times of 8 and 5 months, respectively. Histology significantly influenced outcomes. Median OS was 8 months for patients with GBM, 16 months for patients with grade 3 tumors, and 22 months for patients with low-grade gliomas; respective median PFS times were 5, 8, and 12 months.
Symptomatic brain necrosis is a serious late consequence of reirradiation, with an incidence ranging from 0 to 24.4% at 1 year (Table 1). The reported risk of radionecrosis is < 10% for cumulative EQD2 < 100–110 Gy and rises to 25% for cumulative EQD2 > 130 Gy using an α/β ratio of 2 Gy for normal health brain. Considering an EQD2 of 60 Gy for the initial standard chemoradiation, this means in clinical practice that reirradiation doses of 15–16 Gy given as single fraction (EQD2 = 63.7–72 Gy) or 25 to 30 Gy delivered in 5 fractions (EQD2 = 43.7–60 Gy) carry an acceptable risk of radionecrosis around 10%, at least for patients with relatively small tumor volumes. A low risk ranging from 0.8 to 6.8% has been observed following reirradiation using conventionally fractionated SRT with a median total dose of 36 Gy in 2-Gy fractions (EQD2 = 36 Gy), even in patients with large target volume around 100 ml or higher, or when using large safety GTV-to-CTV margins up to 10 mm.
Superior survival benefit of reirradiation in combination with systemic therapy remains matter of debate. In a few retrospective studies, the combination of RT with alkylating agents offered longer OS and PFS times compared with RT alone, but this benefit seems to be limited to MGMT methylated tumors [31, 37,38,39]. In contrast, other few series failed to show significant survival benefit with the addition of chemotherapy to RT [36, 53, 61]. Retrospective studies observed significantly longer OS with the addition of bevacizumab to SRS and SRT compared to reirradiation alone [33, 48, 51, 62]. Another controversial issue is the potential superiority of combining systemic therapy with reirradiation versus systemic treatment alone [59, 63]. In the secondary analysis of NRG Oncology/RTOG trial 0525 evaluating dose-dense versus standard dose temozolomide in newly diagnosed glioblastoma, Shi et al. [63] investigated the impact of different salvage treatments in 637 patients with recurrent or progressive GBM. Median survival times were 12.2, 8.2, 10.6, 4.8 months, respectively, in patients receiving bevacizumab (44%), reirradiation alone (4%), combined radiation and systemic therapy (10%), or no treatment (42%). Although patients receiving no salvage treatment had significantly lower survival than the others, survival analysis failed to show significant differences among patient groups who received bevacizumab with or without reirradiation. In the NRG Oncology/RTOG 1205 phase II randomized trial of 182 patients with recurrent glioblastoma who received hypofractionated SRT (35 Gy in 3.5 Gy fractions) and concurrent bevacizumab or bevacizumab alone, Tsien et al. [59] observed similar median survival times of 10.1 and 9.7 between groups; however, the combined treatment was associated with better 6-month PFS (54% versus 29%, p < 0.001). The treatment was well tolerated with few (5%) acute and no delayed grade ≥ 3 toxicity, confirming the safety of reirradiation with modern RT techniques.
In summary, reirradiation is a feasible treatment option in selected patients with recurrent diffuse gliomas. An appropriate patient selection is essential to achieve survival benefit. According to international recommendations and prognostic score indexes, reirradiation should be considered in young patients with good performance status, and at least 6 months interval from the first course of RT [61, 64,65,66,67,68]. Survival benefit is longer in patients with lower grade gliomas compared with glioblastoma. Choosing the appropriate radiation technique according to tumor size and location is a key factor in the management of these patients to achieve better clinical outcomes while limiting the potential toxicity. SRS given in one or few fractions can be recommended for small to moderate targets up to 3-3.5 cm in size, while fractionated SRT using doses of 1.8 to 3.5 Gy per fraction should be preferred for larger tumors, especially those close to eloquent structures. Although the combination of reirradiation and bevacizumab did not significantly improve OS for patients with recurrent glioblastoma in NRG Oncology/RTOG1205, the meaningful improvement in the 6-month PFS rate with combined treatment remains an important goal which is clinically beneficial in this disease with limited treatment options. The potential superiority of combining a second course of RT with alkylating agent lomustine (the standard systemic treatment for recurrent glioblastoma in Europe) over lomustine alone will be evaluated in a prospective randomized EORTC phase III trial (LEGATO trial) which will start enrolling patients in Q1 2024 in Europe.
Ependymoma
Ependymomas are rare CNS tumors of neuroectodermal origin that can affect both the pediatric and adult populations, with about 15% of all patients being children of less than 5 years of age [69]. Maximal safe resection followed by adjuvant RT to the tumor bed represents the standard of care [70]. Recurrent disease may occur in 30–50% of patients and it is treated by local excision plus reirradiation as systemic therapies have proven to be of a little benefit. Reirradiation given as focal treatment or CSI RT has been associated with survival benefit [71,72,73,74,75,76,77,78,79] (Table 2).
Tsang et al. (2018) [75] evaluated 101 patients with recurrent ependymoma treated with a second course of fractionated RT after prior focal RT given to a dose of 54 Gy in 1.8 Gy daily fractions. Recurrent tumors received a median dose of 39.6 Gy delivered in 1.8 Gy daily fractions to sites of gross or resected recurrent tumor using either photons (n = 88) or protons (n = 13); 55 patients with recurrent ependymoma were treated with CSI. With a median interval of 26.8 months between the two courses of RT, median durations of OS and freedom from progression were 75.1 and 27.3 months, respectively; and 2-year OS and freedom from progression rates were 74.9% and 53.3%, respectively. CSI was associated with improved outcome, whereas male sex, anaplastic histology at recurrence, treatment group, and a short interval between RT courses were associated with a worse outcome. Grade 1–3 radiation necrosis occurred in 25 patients, with a 10-year cumulative incidence of 26.9%, being of grade ≥ 3 in seven patients (7.9% at 10 years). A similar local control was reported in other retrospective series using both single-fraction SRS (15–24 Gy) or three-fractions SRS (7–8 Gy per fraction), although with an increased risk of radionecrosis up to 50% compared with conventionally fractionated schedules [72, 79, 80].
The question whether CSI as part of reirradiation could improve the clinical outcome compared with focal reirradiation has been addressed in a retrospective study conducted at the Hospital for Sick Children and Princess Margaret Cancer Center in Toronto between 1999 and 2018 [79]. Patients with locally recurrent ependymoma treated before 2012 received focal reirradiation whereas those treated from 2012 received CSI, 23.4–36 Gy in 1.8 Gy daily fractions, followed by boost to the site of resected/macroscopic disease. Among 22 patients with local failure after the first course of RT, the use of CSI as reirradiation was associated with significant improvement in time to recurrence; median time and 5-year rate of time to recurrence were 26.7 months and 15.2% in those who did not receive CSI, respectively, versus not reached and 83.3% for those who received CSI (p = 0.03). However, this difference did not translate into a statistically significant OS difference maybe because of the small number of patients. The treatment was safe, with only one patient who developed grade 3 radionecrosis.
In summary, reirradiation of ependymoma represents an effective treatment approach in patients with locally recurrent lesions after failure of previous adjuvant focal irradiation. The use of single-fraction SRS has been associated with an increased risk of adverse effects compared with conventionally fractionated schedules, especially for larger intact or resected tumors. Preliminary data suggest that CSI as a component of reirradiation offers a statistically significant PFS benefit compared with focal reirradiation, although large series with long-term follow-up are needed to confirm its survival benefit. There is published evidence supporting the use of proton beam therapy for its potential ability of reducing late toxicity in patients receiving CSI [81,82,83,84]. In this regard, results of a prospective study of surgery and fractionated re-irradiation with photon or proton RT in patients for recurrent ependymoma are expected in 2028 (ClinicalTrials.gov, number NCT02125786).
Diffuse midline gliomas
Diffuse midline gliomas H3 K27 altered (previously called diffuse intrinsic pontine gliomas - DIPGs) are extremely aggressive WHO grade IV tumors and represents a leading cause of brain tumor deaths in children, with 90% of children dying within 2 years from the initial diagnosis. According to the Fifth WHO Classification published in 2021 [85], diffuse midline gliomas are characterized by diffuse infiltrative growth in the brain tissue, involvement of midline structures (thalamus, brain stem and spinal cord) and harbor H3 K27M-mutation. RT, using 54–60 Gy in 1.8-2.0 Gy fractions remains the standard of care, but its role is mainly palliative and provides only temporary relief [86, 87].
Few studies investigated clinical outcomes of patients with recurrent/progressive diffuse midline glioma treated with reirradiation [88,89,90,91,92,93]. Using median doses for reirradiation of 18–24 Gy in 1.8-2.0 Gy daily fractions, median OS reported in six published studies ranges from 4 to 8.3 months and median PFS from 3 to 4.5 months from the time of reirradiation (Table 3). A retrospective European study has evaluated benefit and toxicity of reirradiation in 31 patients with diffuse midline glioma at first progression [89]. Most patients were treated with a conventionally fractionated regimen up to a total dose of 20 Gy in 1.8-2.0 Gy daily fractions, given alone or in combination with systemic therapy. Following reirradiation, the reported median survival time was 6.4 months compared to 3 months in a historical cohort of 39 patients receiving no treatment at time of progression (median survival of 13.7 versus 10.3 months after upfront RT). In addition, a clinical improvement was noted in nearly 80% of the patients with no life-threatening or fatal toxicities observed during the follow-up. Longer interval between RT courses was an independent factor for longer survival; in contrast, the addition of systemic therapy and age did not influence survival. In another Canadian retrospective study including 14 patients with diffuse midline glioma who received focal reirradiation using doses of 21.6 to 36 Gy given in 1.8 Gy daily fractions, median OS from reirradiation was 6.5 months compared to 3 months in historical cohorts of 46 patients not treated with reirradiation [91]. Similar OS benefit of a second course of fractionated RT have been confirmed in other few small retrospective series [92, 93].
No severe neurotoxicity related to reirradiation has been observed using total doses < 24 Gy (1.8-2.0 Gy per fraction) [90,91,92]. Amsbaugh et al. [92] evaluated imaging changes, clinical symptoms, and patient- or family-reported quality of life in a prospective phase I/II trial of diffuse midline glioma receiving reirradiation at the time of tumor progression. From the start of reirradiation, median PFS and OS time were 4.5 and 5.8 months, respectively. Six patients who received 24 Gy in 12 fractions and 2 out of 4 patients who received 26.4 Gy in 12 fractions demonstrated improvement in clinical symptoms and quality of life without grade 3 toxicity. In 2 patients who received 30.4 Gy in 14 fractions, grade 3 toxicity occurred in one patient.
In summary, a second course of RT can be considered in children with DPG H3 K27 altered. A few studies demonstrated a median survival of 5 to 7 months following reirradiation, although all data come from retrospective series. Clinical benefit can be observed in up to 80% of patients and this has been associated with an improvement in quality of life. Severe toxicities from reirradiation appear to be limited using conventionally fractionated RT schedules with doses of 20 to 24 Gy. Regarding the timing of reirradiation, interval of at least 6 months between the two radiation treatments is associated with better outcome. Future clinical trials need to assess optimal dose, fractionation, interval between treatments, and the concurrent use of systemic agents in such patients.
Brain metastases
SRS is the recommended treatment for patients with a limited number of brain metastases (1–4 lesions), resulting in a significant decrease of neurocognitive decline compared to WBRT without detrimental effects on OS [94, 95]. In the recent ESMO-EANO and ASTRO guidelines on treatment of brain metastases [96, 97], SRS has been also recommended for patients with 5–10 lesions. The reported local control following SRS is around 75 to 90% at one year, with late local recurrences that are increasingly observed. For patients with locally recurrent brain metastases, repeat SRS is a challenging treatment because of the difficulty of discerning progression from treatment effect and the increased risk of radionecrosis. A summary of selected reirradiation studies for brain metastases is shown in Table 4 [98,99,100,101,102,103,104,105]. With a variable median follow-up of 7–19 months, local control ranges between 70% and 95% at 1 year and the risk of symptomatic radionecrosis is around 7–16% for 546 patients included in eight selected studies (Table 4).
Sneed et al. [105] evaluated the efficacy and safety of repeat single-fraction SRS in terms of treatment failure and risk of adverse radiation effects for 124 patients with 229 recurrent brain metastases from various cancer types, the most common from breast cancer, lung cancer, and melanoma. With a median SRS prescription dose of 18 Gy and median follow-up of 14.5 months, the 1-year freedom from progression was 82% and risk of symptomatic adverse radiation effects 11% for lesions with a quadratic mean diameter of 0.75-2.0 cm. For lesions with a quadratic mean diameter of 2.01-3.0 cm, SRS was associated with 1-year control rates of 65% and a higher risk of symptomatic adverse radiation effects of 24%. In another multi-institutional retrospective series of 102 patients with 123 brain metastases treated with repeat SRS after local or marginal recurrence after prior SRS, Kowalchuk et al. [103] reported 1-year local control rates of 79% and 1-year incidence rates of symptomatic adverse radiation effects of 7%. Tumor control was significantly better for lesion ≤1 cm (p < 0.005). The risk of symptomatic radionecrosis was higher for cumulative maximum doses ≥ 40 Gy or for a volume of normal brain receiving 12 Gy > 9 cm3 at the time of repeat SRS (p < 0.025). Similar 1-year local control rates of 70–80% and symptomatic radionecrosis rates around 7–16% have been shown in other published series of repeat SRS after either prior SRS or WBRT [98, 101].
Fractionated SRS (2–5 fractions) has been suggested as an alternative treatment option to single-fraction SRS for locally recurrent brain metastases [99, 100, 104, 106]. In a systematic review and meta-analysis of stereotactic reirradiation for local failure of brain metastases following previous SRS, Loi et al. [106] reported clinical outcomes for 335 patients with 389 brain metastases treated with either single-fraction (n = 282) or fractionated (n = 107) SRS. With a median follow-up of 12 months from the time of repeat SRS, median OS time was 14 months, 1-year local failure was 24%, and crude cumulative incidence of radiation necrosis was 13%. There were no differences in local control and risk of symptomatic adverse radiation effects with single-fraction SRS using doses of 16–19 Gy or fractionated SRS using a total dose of 21–24 Gy given in 3 fractions, although the median volume of lesions receiving 3-fractions SRS was generally larger. No factors were associated with an increased risk of radionecrosis, including tumor volume, histology, higher biological effective dose, and longer time interval from first SRS.
Overall, repeat SRS has emerged as an effective strategy for patients with recurrent brain metastases. In the respect of the relatively small number of patients reported and retrospective nature of studies, either single-fraction or fractionated SRS are associated with high local control and acceptable risk of symptomatic radionecrosis < 15%. Optimal radiation dose and fractionation for different target volumes, as well safe combination with systemic agents need to be defined.
Other brain tumors
Reirradiation has been used as possible salvage treatment option for several other recurrent brain tumors. In patients with recurrent medulloblastoma after standard CSI and a boost to the posterior fossa/tumor bed, small retrospective studies observed survival rates of 50–75% at 1 year following a second course of RT, with higher rates for those presenting with focal recurrences versus diffuse leptomeningeal disease [107,108,109,110,111]. Hypofractionated schedules (25–30 Gy in 3 to 10 fractions) are typically used for focal radiation while a dose of 20–24 Gy in 1.8 Gy daily fractions is used to the entire spine. Single site of recurrence, minimal residual disease, time from the first course of RT, and molecular subtypes are known to affect survival [112]. The risk of toxicity is low when cumulative EQD2 for brain and spine does not exceed 150 Gy and 120 Gy [7, 8]. Few published series suggest that a second course of RT, both SRS and SRT using either photons or protons, may be a feasible salvage treatment option for selected patients with skull base recurrent tumors, including recurrent aggressive pituitary adenomas [113, 114] and meningiomas [115,116,117], which is associated with a risk of symptomatic radionecrosis, cranial deficits, and radiation-induced optic neuropathy < 15%.
Conclusions
This review represents a synthesis of the available literature data and a basis for further consideration. An increasing number of studies indicates that reirradiation is a feasible treatment option among patients with recurrent brain tumors. Although caution is required when performing a second course or RT for the increased risk of radiation-induced toxicity, most studies using modern radiation techniques indicate that retreatment is associated with a risk of adverse radiation effects < 10% for cumulative EQD2 doses of 100 to 110 Gy in patients with recurrent brain lesion and for cumulative EQD2 doses of 70 to 75 Gy in those with recurrent spine lesions assuming an α/β ratio of 2 Gy for normal tissue. While available data support the use of reirradiation as salvage therapy in selected patients with brain tumors, a definitive judgment on the efficacy and safety of a second course of RT and its superiority over other treatment options (systemic treatment or repeat surgery) cannot be made because of the small number of patients and the retrospective nature of most studies. Only prospective studies with appropriate follow-up can confirm OS benefit of reirradiation for different tumors, as well to address unanswered questions such as optimal radiation dose and fractionation, target volumes delineation, combination of reirradiation with new systemic agents and immunotherapy, and which patients will benefit most from treatment. Until these data become available, the decision to offer reirradiation in clinical practice to patients with recurrent tumors to improve disease control and OS should be always weighed against the potential toxicity of treatment.
References
Minniti G, Goldsmith C, Brada M (2012) Chap. 16 - Radiotherapy. In: Handbook of clinical neurology, 104, 215–228. https://doi.org/10.1016/B978-0-444-52138-5.00016-5
Scaringi C, Agolli L, Minniti G (2018) Technical advances in Radiation Therapy for Brain Tumors. Anticancer Res 38(11):6041–6045. https://doi.org/10.21873/anticanres.12954
Combs SE, Kessel K, Habermehl D, Haberer T, Jäkel O, Debus J (2013) Proton and carbon ion radiotherapy for primary brain tumors and tumors of the skull base. Acta Oncol (Stockholm Sweden) 52(7):1504–1509. https://doi.org/10.3109/0284186X.2013.818255
Ang KK, Jiang GL, Feng Y, Stephens LC, Tucker SL, Price RE (2001) Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys 50(4):1013–1020. https://doi.org/10.1016/s0360-3016(01)01599-1
Medin PM, Foster RD, van der Kogel AJ, Sayre JW, McBride WH, Solberg TD (2012) Spinal cord tolerance to reirradiation with single-fraction radiosurgery: a swine model. Int J Radiat Oncol Biol Phys 83(3):1031–1037. https://doi.org/10.1016/j.ijrobp.2011.08.030
Mayer R, Sminia P (2008) Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 70(5):1350–1360. https://doi.org/10.1016/j.ijrobp.2007.08.015
Nieder C, Grosu AL, Andratschke NH, Molls M (2006) Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys 66(5):1446–1449. https://doi.org/10.1016/j.ijrobp.2006.07.1383
Kirkpatrick JP, van der Kogel AJ, Schultheiss TE (2010) Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 76(3 Suppl):42–S49. https://doi.org/10.1016/j.ijrobp.2009.04.095
Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, Dicker AP (2010) Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys 76(3 Suppl):S20–S27. https://doi.org/10.1016/j.ijrobp.2009.02.091
Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiation Oncol (London England) 6:48. https://doi.org/10.1186/1748-717X-6-48
Milano MT, Grimm J, Niemierko A, Soltys SG, Moiseenko V, Redmond KJ, Yorke E, Sahgal A, Xue J, Mahadevan A, Muacevic A, Marks LB, Kleinberg LR (2021) Single- and multifraction stereotactic radiosurgery Dose/Volume tolerances of the brain. Int J Radiat Oncol Biol Phys 110(1):68–86. https://doi.org/10.1016/j.ijrobp.2020.08.013
Mayo C, Yorke E, Merchant TE (2010) Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys 76(3 Suppl):36–S41. https://doi.org/10.1016/j.ijrobp.2009.08.078
Lambrecht M, Eekers DBP, Alapetite C, Burnet NG, Calugaru V, Coremans IEM, Fossati P, Høyer M, Langendijk JA, Méndez Romero A, Paulsen F, Perpar A, Renard L, de Ruysscher D, Timmermann B, Vitek P, Weber DC, van der Weide HL, Whitfield GA, Wiggenraad R, Roelofs E, Nyström PW, Troost EGC (2018) Radiation dose constraints for organs at risk in neuro-oncology; the European Particle Therapy Network consensus. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 128(1):26–36. https://doi.org/10.1016/j.radonc.2018.05.001. work package 1 of the taskforce “European Particle Therapy Network” of ESTRO
Combs SE, Baumert BG, Bendszus M, Bozzao A, Brada M, Fariselli L, Fiorentino A, Ganswindt U, Grosu AL, Lagerwaard FL, Niyazi M, Nyholm T, Paddick I, Weber DC, Belka C, Minniti G (2021) ESTRO ACROP guideline for target volume delineation of skull base tumors. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 156:80–94. https://doi.org/10.1016/j.radonc.2020.11.014
Leber KA, Berglöff J, Pendl G (1998) Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg 88(1):43–50. https://doi.org/10.3171/jns.1998.88.1.0043
Tishler RB, Loeffler JS, Lunsford LD, Duma C, Alexander E 3rd, Kooy HM, Flickinger JC (1993) Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys 27(2):215–221. https://doi.org/10.1016/0360-3016(93)90230-s
Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T (2013) Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg 118(3):557–565. https://doi.org/10.3171/2012.10.JNS12523
Leavitt JA, Stafford SL, Link MJ, Pollock BE (2013) Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 87(3):524–527. https://doi.org/10.1016/j.ijrobp.2013.06.2047
Pollock BE, Link MJ, Leavitt JA, Stafford SL (2014) Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery 75(4):456–460. https://doi.org/10.1227/NEU.0000000000000457
Timmerman RD (2008) An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol 18(4):215–222. https://doi.org/10.1016/j.semradonc.2008.04.001
Timmerman R (2022) A story of Hypofractionation and the table on the Wall. Int J Radiat Oncol Biol Phys 112(1):4–21. https://doi.org/10.1016/j.ijrobp.2021.09.027
Sminia P, Mayer R (2012) External beam radiotherapy of recurrent glioma: radiation tolerance of the human brain. Cancers 4(2):379–399. https://doi.org/10.3390/cancers4020379
Minniti G, Niyazi M, Alongi F, Navarria P, Belka C (2021) Current status and recent advances in reirradiation of glioblastoma. Radiation Oncol (London England) 16(1):36. https://doi.org/10.1186/s13014-021-01767-9
Niyazi M, Karin I, Söhn M, Nachbichler SB, Lang P, Belka C, Ganswindt U (2013) Analysis of equivalent uniform dose (EUD) and conventional radiation treatment parameters after primary and re-irradiation of malignant glioma. Radiation Oncol (London England) 8:287. https://doi.org/10.1186/1748-717X-8-287
Lu VM, Welby JP, Mahajan A, Laack NN, Daniels DJ (2019) Reirradiation for diffuse intrinsic pontine glioma: a systematic review and meta-analysis. Child’s Nerv system: ChNS: official J Int Soc Pediatr Neurosurg 35(5):739–746. https://doi.org/10.1007/s00381-019-04118-y
Seidensaal K, Harrabi SB, Uhl M, Debus J (2020) Re-irradiation with protons or heavy ions with focus on head and neck, skull base and brain malignancies. Br J Radiol 93(1107):20190516. https://doi.org/10.1259/bjr.20190516
Amichetti M, Amelio D, Minniti G (2012) Radiosurgery with photons or protons for benign and malignant tumours of the skull base: a review. Radiation Oncol (London England) 7:210. https://doi.org/10.1186/1748-717X-7-210
Castellano A, Bailo M, Cicone F, Carideo L, Quartuccio N, Mortini P, Falini A, Cascini GL, Minniti G (2021) Advanced Imaging Techniques for Radiotherapy Planning of Gliomas. Cancers 13(5):1063. https://doi.org/10.3390/cancers13051063
Combs SE, Widmer V, Thilmann C, Hof H, Debus J, Schulz-Ertner D (2005) Stereotactic radiosurgery (SRS): treatment option for recurrent glioblastoma multiforme (GBM). Cancer 104(10):2168–2173. https://doi.org/10.1002/cncr.21429
Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D (2005) Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin oncology: official J Am Soc Clin Oncol 23(34):8863–8869. https://doi.org/10.1200/JCO.2005.03.4157
Grosu AL, Weber WA, Franz M, Stärk S, Piert M, Thamm R, Gumprecht H, Schwaiger M, Molls M, Nieder C (2005) Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 63(2):511–519. https://doi.org/10.1016/j.ijrobp.2005.01.056
Kong DS, Lee JI, Park K, Kim JH, Lim DH, Nam DH (2008) Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer 112(9):2046–2051. https://doi.org/10.1002/cncr.23402
Cuneo KC, Vredenburgh JJ, Sampson JH, Reardon DA, Desjardins A, Peters KB, Friedman HS, Willett CG, Kirkpatrick JP (2012) Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 82(5):2018–2024. https://doi.org/10.1016/j.ijrobp.2010.12.074
Fokas E, Wacker U, Gross MW, Henzel M, Encheva E, Engenhart-Cabillic R (2009) Hypofractionated stereotactic reirradiation of recurrent glioblastomas: a beneficial treatment option after high-dose radiotherapy. Strahlenther Onkol 185(4):235–240. https://doi.org/10.1007/s00066-009-1753-x
Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, Lymberis S, Yamada Y, Chang J, Abrey LE (2009) Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75(1):156–163. https://doi.org/10.1016/j.ijrobp.2008.10.043
Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, Evans JJ, Hyslop T, Pequignot E, Downes B, Comber E, Maltenfort M, Dicker AP, Werner-Wasik M (2010) Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin oncology: official J Am Soc Clin Oncol 28(18):3048–3053. https://doi.org/10.1200/JCO.2009.25.6941
Minniti G, Armosini V, Salvati M, Lanzetta G, Caporello P, Mei M, Osti MF, Maurizi RE (2011) Fractionated stereotactic reirradiation and concurrent temozolomide in patients with recurrent glioblastoma. J Neurooncol 103(3):683–691. https://doi.org/10.1007/s11060-010-0446-8
Minniti G, Scaringi C, De Sanctis V, Lanzetta G, Falco T, Di Stefano D, Esposito V, Enrici RM (2013) Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol 111(2):187–194. https://doi.org/10.1007/s11060-012-0999-9
Greenspoon JN, Sharieff W, Hirte H, Overholt A, Devillers R, Gunnarsson T, Whitton A (2014) Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: a prospective cohort study. OncoTargets and therapy 7:485–490. https://doi.org/10.2147/OTT.S60358
Martínez-Carrillo M, Tovar-Martín I, Zurita-Herrera M, Del Moral-Ávila R, Guerrero-Tejada R, Saura-Rojas E, Osorio-Ceballos JL, Arrebola-Moreno JP, Expósito-Hernández J (2014) Salvage radiosurgery for selected patients with recurrent malignant gliomas. BioMed research international, 2014, 657953. https://doi.org/10.1155/2014/657953
Wick W, Fricke H, Junge K, Kobyakov G, Martens T, Heese O, Wiestler B, Schliesser MG, von Deimling A, Pichler J, Vetlova E, Harting I, Debus J, Hartmann C, Kunz C, Platten M, Bendszus M, Combs SE (2014) A phase II, randomized, study of weekly APG101 + reirradiation versus reirradiation in progressive glioblastoma. Clin cancer research: official J Am Association Cancer Res 20(24):6304–6313. https://doi.org/10.1158/1078-0432.CCR-14-0951-T
Kim HR, Kim KH, Kong DS, Seol HJ, Nam DH, Lim DH, Lee JI (2015) Outcome of salvage treatment for recurrent glioblastoma. J Clin neuroscience: official J Neurosurgical Soc Australasia 22(3):468–473. https://doi.org/10.1016/j.jocn.2014.09.018
Minniti G, Agolli L, Falco T, Scaringi C, Lanzetta G, Caporello P, Osti MF, Esposito V, Enrici RM (2015) Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. J Neurooncol 122(3):559–566. https://doi.org/10.1007/s11060-015-1745-x
Pinzi V, Orsi C, Marchetti M, Milanesi IM, Bianchi LC, DiMeco F, Cuccarini V, Farinotti M, Ferroli P, Finocchiaro G, Franzini A, Fumagalli M, Silvani A, Fariselli L (2015) Radiosurgery reirradiation for high-grade glioma recurrence: a retrospective analysis. Neurol sciences: official J Italian Neurol Soc Italian Soc Clin Neurophysiol 36(8):1431–1440. https://doi.org/10.1007/s10072-015-2172-7
Frischer JM, Marosi C, Woehrer A, Hainfellner JA, Dieckmann KU, Eiter H, Wang WT, Mallouhi A, Ertl A, Knosp E, Filipits M, Kitz K, Gatterbauer B (2016) Gamma Knife Radiosurgery in Recurrent Glioblastoma. Stereotact Funct Neurosurg 94(4):265–272. https://doi.org/10.1159/000448924
Imber BS, Kanungo I, Braunstein S, Barani IJ, Fogh SE, Nakamura JL, Berger MS, Chang EF, Molinaro AM, Cabrera JR, McDermott MW, Sneed PK, Aghi MK (2017) Indications and efficacy of Gamma Knife Stereotactic Radiosurgery for recurrent glioblastoma: 2 decades of institutional experience. Neurosurgery 80(1):129–139. https://doi.org/10.1227/NEU.0000000000001344
Kim BS, Kong DS, Seol HJ, Nam DH, Lee JI (2017) MGMT promoter methylation status as a prognostic factor for the outcome of gamma knife radiosurgery for recurrent glioblastoma. J Neurooncol 133(3):615–622. https://doi.org/10.1007/s11060-017-2478-9
Schnell O, Thorsteinsdottir J, Fleischmann DF, Lenski M, Abenhardt W, Giese A, Tonn JC, Belka C, Kreth FW, Niyazi M (2016) Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol 130(3):591–599. https://doi.org/10.1007/s11060-016-2267-x
Sharma M, Schroeder JL, Elson P, Meola A, Barnett GH, Vogelbaum MA, Suh JH, Chao ST, Mohammadi AM, Stevens GHJ, Murphy ES, Angelov L (2018) Outcomes and prognostic stratification of patients with recurrent glioblastoma treated with salvage stereotactic radiosurgery. J Neurosurg 131(2):489–499. https://doi.org/10.3171/2018.4.JNS172909
Palmer JD, Bhamidipati D, Song A, Eldredge-Hindy HB, Siglin J, Dan TD, Champ CE, Zhang I, Bar-Ad V, Kim L, Glass J, Evans JJ, Andrews DW, Werner-Wasik M, Shi W (2018) Bevacizumab and re-irradiation for recurrent high grade gliomas: does sequence matter? J Neurooncol 140(3):623–628. https://doi.org/10.1007/s11060-018-2989-z
Fleischmann DF, Jenn J, Corradini S, Ruf V, Herms J, Forbrig R, Unterrainer M, Thon N, Kreth FW, Belka C, Niyazi M (2019) Bevacizumab reduces toxicity of reirradiation in recurrent high-grade glioma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 138:99–105. https://doi.org/10.1016/j.radonc.2019.06.009
Morris SL, Zhu P, Rao M, Martir M, Zhu JJ, Hsu S, Ballester LY, Day AL, Tandon N, Kim DH, Shepard S, Blanco A, Esquenazi Y (2019) Gamma Knife Stereotactic Radiosurgery in Combination with Bevacizumab for recurrent glioblastoma. World Neurosurg 127:e523–e533. https://doi.org/10.1016/j.wneu.2019.03.193
Navarria P, Minniti G, Clerici E, Tomatis S, Pinzi V, Ciammella P, Galaverni M, Amelio D, Scartoni D, Scoccianti S, Krengli M, Masini L, Draghini L, Maranzano E, Borzillo V, Muto P, Ferrarese F, Fariselli L, Livi L, Pasqualetti F, Fiorentino A, Alongi F, di Buglione M, Magrini S, Scorsetti M (2019) Re-irradiation for recurrent glioma: outcome evaluation, toxicity and prognostic factors assessment. A multicenter study of the Radiation Oncology Italian Association (AIRO). J Neurooncol 142(1):59–67. https://doi.org/10.1007/s11060-018-03059-x
Kaul D, Pudlitz V, Böhmer D, Wust P, Budach V, Grün A (2020) Reirradiation of high-grade gliomas: a retrospective analysis of 198 patients based on the Charité Data Set. Adv Radiat Oncol 5:959–964. https://doi.org/10.1016/j.adro.2020.06.005
Kim MS, Lim J, Shin HS, Cho KG (2020) Re-Irradiation and its contribution to good prognosis in recurrent glioblastoma patients. Brain tumor research and treatment 8(1):29–35. https://doi.org/10.14791/btrt.2020.8.e10
Saeed AM, Khairnar R, Sharma AM, Larson GL, Tsai HK, Wang CJ, Halasz LM, Chinnaiyan P, Vargas CE, Mishra MV (2020) Clinical outcomes in patients with recurrent glioblastoma treated with Proton Beam Therapy Reirradiation: analysis of the multi-institutional Proton Collaborative Group Registry. Adv radiation Oncol 5(5):978–983. https://doi.org/10.1016/j.adro.2020.03.022
Scartoni D, Amelio D, Palumbo P, Giacomelli I, Amichetti M (2020) Proton therapy re-irradiation preserves health-related quality of life in large recurrent glioblastoma. J Cancer Res Clin Oncol 146(6):1615–1622. https://doi.org/10.1007/s00432-020-03187-w
Attia AM, Farrag A, Farouk BR, Attia NM (2022) Outcome and prognostic factors in recurrent Glioblastoma Multiforme treated with re-irradiation: a retrospective study from a Tertiary Care Hospital. SECI Oncol J 10(3):152–161
Tsien CI, Pugh SL, Dicker AP, Raizer JJ, Matuszak MM, Lallana EC, Huang J, Algan O, Deb N, Portelance L, Villano JL, Hamm JT, Oh KS, Ali AN, Kim MM, Lindhorst SM, Mehta MP (2023) NRG Oncology/RTOG1205: a randomized phase II trial of concurrent Bevacizumab and Reirradiation Versus Bevacizumab alone as treatment for recurrent glioblastoma. J Clin oncology: official J Am Soc Clin Oncol 41(6):1285–1295. https://doi.org/10.1200/JCO.22.00164
Kazmi F, Soon YY, Leong YH, Koh WY, Vellayappan B (2019) Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neurooncol 142(1):79–90. https://doi.org/10.1007/s11060-018-03064-0
Combs SE, Niyazi M, Adeberg S, Bougatf N, Kaul D, Fleischmann DF, Gruen A, Fokas E, Rödel CM, Eckert F, Paulsen F, Oehlke O, Grosu AL, Seidlitz A, Lattermann A, Krause M, Baumann M, Guberina M, Stuschke M, Budach V, Belka C, Debus J, Kessel KA (2018) Re-irradiation of recurrent gliomas: pooled analysis and validation of an established prognostic score-report of the Radiation Oncology Group (ROG) of the German Cancer Consortium (DKTK). Cancer Med 7(5):1742–1749. https://doi.org/10.1002/cam4.1425
Flieger M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, la Fougère C, Ertl L, Linn J, Herrlinger U, Belka C, Niyazi M (2014) Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol 117(2):337–345. https://doi.org/10.1007/s11060-014-1394-5
Shi W, Scannell Bryan M, Gilbert MR, Mehta MP, Blumenthal DT, Brown PD, Valeinis E, Hopkins K, Souhami L, Andrews DW, Tzuk-Shina T, Howard SP, Youssef EF, Lessard N, Dignam JJ, Werner-Wasik M (2018) Investigating the Effect of Reirradiation or systemic therapy in patients with Glioblastoma after Tumor Progression: a secondary analysis of NRG Oncology/Radiation Therapy Oncology Group Trial 0525. Int J Radiat Oncol Biol Phys 100(1):38–44. https://doi.org/10.1016/j.ijrobp.2017.08.038
Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, Schlegel J, Meyer B, Combs SE (2017) Validation of an established prognostic score after re-irradiation of recurrent glioma. Acta Oncol (Stockholm Sweden) 56(3):422–426. https://doi.org/10.1080/0284186X.2016.1276621
Sulman EP, Ismaila N, Chang SM (2017) Radiation Therapy for Glioblastoma: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the American Society for Radiation Oncology Guideline. J Oncol Pract 13(2):123–127. https://doi.org/10.1200/JOP.2016.018937
Krauze AV, Peters C, Cheng J, Ning H, Mackey M, Rowe L, Cooley-Zgela T, Smart DD, Camphausen K (2017) Re-irradiation for recurrent glioma- the NCI experience in tumor control, OAR toxicity and proposal of a novel prognostic scoring system. Radiation Oncol (London England) 12(1):191. https://doi.org/10.1186/s13014-017-0930-9
Niyazi M, Adeberg S, Kaul D, Boulesteix AL, Bougatf N, Fleischmann DF, Grün A, Krämer A, Rödel C, Eckert F, Paulsen F, Kessel KA, Combs SE, Oehlke O, Grosu AL, Seidlitz A, Lattermann A, Krause M, Baumann M, Guberina M, Stuschke M, Budach V, Belka C, Debus J (2018) Independent validation of a new reirradiation risk score (RRRS) for glioma patients predicting post-recurrence survival: a multicenter DKTK/ROG analysis. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 127(1):121–127. https://doi.org/10.1016/j.radonc.2018.01.011
Chapman CH, Hara JH, Molinaro AM, Clarke JL, Oberheim Bush NA, Taylor JW, Butowski NA, Chang SM, Fogh SE, Sneed PK, Nakamura JL, Raleigh DR, Braunstein SE (2019) Reirradiation of recurrent high-grade glioma and development of prognostic scores for progression and survival. Neuro-oncology Pract 6(5):364–374. https://doi.org/10.1093/nop/npz017
Parkin DM, Stiller CA, Draper GJ, Bieber CA (1988) The international incidence of childhood cancer. Int J Cancer 42(4):511–520. https://doi.org/10.1002/ijc.2910420408
Rudà R, Reifenberger G, Frappaz D, Pfister SM, Laprie A, Santarius T, Roth P, Tonn JC, Soffietti R, Weller M, Moyal EC (2018) EANO guidelines for the diagnosis and treatment of ependymal tumors. Neurooncology 20(4):445–456. https://doi.org/10.1093/neuonc/nox166
Merchant TE, Boop FA, Kun LE, Sanford RA (2008) A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys 71(1):87–97. https://doi.org/10.1016/j.ijrobp.2007.09.037
Hoffman LM, Plimpton SR, Foreman NK, Stence NV, Hankinson TC, Handler MH, Hemenway MS, Vibhakar R, Liu AK (2014) Fractionated stereotactic radiosurgery for recurrent ependymoma in children. J Neurooncol 116(1):107–111. https://doi.org/10.1007/s11060-013-1259-3
Lobón MJ, Bautista F, Riet F, Dhermain F, Canale S, Dufour C, Blauwblomme T, Zerah M, Beccaria K, Saint-Rose C, Puget S, Carrie C, Lartigau E, Bondiau PY, Valteau-Couanet D, Grill J, Bolle S (2016) Re-irradiation of recurrent pediatric ependymoma: modalities and outcomes: a twenty-year survey. SpringerPlus 5(1):879. https://doi.org/10.1186/s40064-016-2562-1
Murray LJ, Hawkins C, Taylor M, Bartels KU, Huang A, Kulkarni A, Ramaswamy V, Bouffet E, Tabori U, Lapierre N (2016) Re-irradiation for relapsed paediatric ependymoma. J Clin Oncol 34:10565–10565
Tsang DS, Burghen E, Klimo P Jr, Boop FA, Ellison DW, Merchant TE (2018) Outcomes after Reirradiation for Recurrent Pediatric Intracranial Ependymoma. Int J Radiat Oncol Biol Phys 100(2):507–515. https://doi.org/10.1016/j.ijrobp.2017.10.002
Tsang DS, Murray L, Ramaswamy V, Zapotocky M, Tabori U, Bartels U, Huang A, Dirks PB, Taylor MD, Hawkins C, Bouffet E, Laperriere N (2019) Craniospinal irradiation as part of re-irradiation for children with recurrent intracranial ependymoma. Neurooncology 21(4):547–557. https://doi.org/10.1093/neuonc/noy191
Régnier E, Laprie A, Ducassou A, Bolle S, Supiot S, Muracciole X, Claude L, Chapet S, Coche-Dequéant B, Vigneron C, Leseur J, Bondiau PY, Habrand JL, Bernier V (2019) Re-irradiation of locally recurrent pediatric intracranial ependymoma: experience of the french society of children’s cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 132:1–7. https://doi.org/10.1016/j.radonc.2018.11.009
Gupta T, Maitre M, Gupta P, Krishnatry R, Chatterjee A, Moiyadi A, Shetty P, Singh V, Chinnaswamy G, Epari S, Sahay A, Patil V, GodaSastri J (2020) Extent of re-excision, sequence/timing of salvage re-irradiation, and disease-free interval impact upon clinical outcomes in recurrent/progressive ependymoma. J Neurooncol 147(2):405–415. https://doi.org/10.1007/s11060-020-03434-7
Mak DY, Laperriere N, Ramaswamy V, Bouffet E, Murray JC, McNall-Knapp RY, Bielamowicz K, Paulino AC, Zaky W, McGovern SL, Okcu MF, Tabori U, Atwi D, Dirks PB, Taylor MD, Tsang DS, Bavle A (2021) Reevaluating surgery and re-irradiation for locally recurrent pediatric ependymoma-a multi-institutional study. Neuro-oncology Adv 3(1):vdab158. https://doi.org/10.1093/noajnl/vdab158
Hodgson DC, Goumnerova LC, Loeffler JS, Dutton S, Black PM, Alexander E 3rd, Xu R, Kooy H, Silver B, Tarbell NJ (2001) Radiosurgery in the management of pediatric brain tumors. Int J Radiat Oncol Biol Phys 50(4):929–935. https://doi.org/10.1016/s0360-3016(01)01518-8
Young S, Phaterpekar K, Tsang DS, Boldt G, Bauman GS (2023) Proton Radiotherapy for Management of Medulloblastoma: a systematic review of clinical outcomes. Adv radiation Oncol 8(4):101189. https://doi.org/10.1016/j.adro.2023.101189
Brown AP, Barney CL, Grosshans DR, McAleer MF, de Groot JF, Puduvalli VK, Tucker SL, Crawford CN, Khan M, Khatua S, Gilbert MR, Brown PD, Mahajan A (2013) Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys 86(2):277–284. https://doi.org/10.1016/j.ijrobp.2013.01.014
Barney CL, Brown AP, Grosshans DR, McAleer MF, de Groot JF, Puduvalli V, Tucker SL, Crawford CN, Gilbert MR, Brown PD, Mahajan A (2014) Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation. Neurooncology 16(2):303–309. https://doi.org/10.1093/neuonc/not155
Song S, Park HJ, Yoon JH, Kim DW, Park J, Shin D, Shin SH, Kang HJ, Kim SK, Phi JH, Kim JY (2014) Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol (Stockholm Sweden) 53(9):1158–1164. https://doi.org/10.3109/0284186X.2014.887225
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the Central Nervous System: a summary. Neurooncology 23(8):1231–1251. https://doi.org/10.1093/neuonc/noab106
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7(3):241–248. https://doi.org/10.1016/S1470-2045(06)70615-5
Langmoen IA, Lundar T, Storm-Mathisen I, Lie SO, Hovind KH (1991) Management of pediatric pontine gliomas. Child’s Nerv system: ChNS: official J Int Soc Pediatr Neurosurg 7(1):13–15. https://doi.org/10.1007/BF00263825
Massimino M, Biassoni V, Miceli R, Schiavello E, Warmuth-Metz M, Modena P, Casanova M, Pecori E, Giangaspero F, Antonelli M, Buttarelli FR, Potepan P, Pollo B, Nunziata R, Spreafico F, Podda M, Anichini A, Clerici CA, Sardi I, De Cecco L, Bode U, Bach F, Gandola L (2014) Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol 118(2):305–312. https://doi.org/10.1007/s11060-014-1428-z
Janssens GO, Gandola L, Bolle S, Mandeville H, Ramos-Albiac M, van Beek K, Benghiat H, Hoeben B, Morales La Madrid A, Kortmann RD, Hargrave D, Menten J, Pecori E, Biassoni V, von Bueren AO, van Vuurden DG, Massimino M, Sturm D, Peters M, Kramm CM (2017) Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: A matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. European journal of cancer (Oxford, England: 1990), 73, 38–47. https://doi.org/10.1016/j.ejca.2016.12.007
Kline C, Liu SJ, Duriseti S, Banerjee A, Nicolaides T, Raber S, Gupta N, Haas-Kogan D, Braunstein S, Mueller S (2018) Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: a single-institution experience. J Neurooncol 140(3):629–638. https://doi.org/10.1007/s11060-018-2991-5
Lassaletta A, Strother D, Laperriere N, Hukin J, Vanan MI, Goddard K, Lafay-Cousin L, Johnston DL, Zelcer S, Zapotocky M, Rajagopal R, Ramaswamy V, Hawkins C, Tabori U, Huang A, Bartels U, Bouffet E (2018) Reirradiation in patients with diffuse intrinsic pontine gliomas: the canadian experience. Pediatr blood cancer 65(6):e26988. https://doi.org/10.1002/pbc.26988
Amsbaugh MJ, Mahajan A, Thall PF, McAleer MF, Paulino AC, Grosshans D, Khatua S, Ketonen L, Fontanilla H, McGovern SL (2019) A phase 1/2 trial of reirradiation for diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys 104(1):144–148. https://doi.org/10.1016/j.ijrobp.2018.12.043
Krishnatry R, Manjali JJ, Chinnaswamy G, Chatterjee A, Goda JS, Janu A, Sahu A, Jalali R, Gupta T (2021) Clinical approach to re-irradiation for recurrent diffuse intrinsic pontine glioma. Jpn J Clin Oncol 51(5):762–768. https://doi.org/10.1093/jjco/hyab006
Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin oncology: official J Am Soc Clin Oncol 29(2):134–141. https://doi.org/10.1200/JCO.2010.30.1655
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG 2nd, Deming R, Burri SH, Ménard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL (2016) Effect of Radiosurgery alone vs Radiosurgery with Whole Brain Radiation Therapy on cognitive function in patients with 1 to 3 brain metastases: a Randomized Clinical Trial. JAMA 316(4):401–409. https://doi.org/10.1001/jama.2016.9839
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, Jordan JT, Lassman AB, Maues J, Mohile N, Redjal N, Stevens G, Sulman E, van den Bent M, Wallace HJ, Weinberg JS, Zadeh G, Schiff D (2022) Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J Clin oncology: official J Am Soc Clin Oncol 40(5):492–516. https://doi.org/10.1200/JCO.21.02314
Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, Galldiks N, de Azambuja E, Berghoff AS, Metellus P, Peters S, Hong YK, Winkler F, Schadendorf D, van den Bent M, Seoane J, Stahel R, Minniti G, Wesseling P, Weller M, Preusser M (2021) EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Annals of oncology: official journal of the European Society for Medical Oncology 32(11):1332–1347. https://doi.org/10.1016/j.annonc.2021.07.016
Terakedis BE, Jensen RL, Boucher K, Shrieve DC (2014) Tumor control and incidence of radiation necrosis after reirradiation with stereotactic radiosurgery for brain metastases. Journal of radiosurgery and SBRT, 3(1), 21–28. PMCID: PMC5725326
Minniti G, Scaringi C, Paolini S, Clarke E, Cicone F, Esposito V, Romano A, Osti M, Enrici RM (2016) Repeated stereotactic radiosurgery for patients with progressive brain metastases. J Neurooncol 126(1):91–97. https://doi.org/10.1007/s11060-015-1937-4
Shultz DB, Modlin LA, Jayachandran P, Von Eyben R, Gibbs IC, Choi CYH, Chang SD, Harsh GR 4th, Li G, Adler JR, Hancock SL, Soltys SG (2015) Repeat courses of stereotactic radiosurgery (SRS), deferring whole-brain irradiation, for New Brain Metastases after initial SRS. Int J Radiat Oncol Biol Phys 92(5):993–999. https://doi.org/10.1016/j.ijrobp.2015.04.036
Balermpas P, Stera S, von der Müller J, Loutfi-Krauss B, Forster MT, Wagner M, Keller C, Rödel C, Seifert V, Blanck O, Wolff R (2018) Repeated in-field radiosurgery for locally recurrent brain metastases: feasibility, results and survival in a heavily treated patient cohort. PLoS ONE 13(6):e0198692. https://doi.org/10.1371/journal.pone.0198692
Jiang X, Wang H, Song Y, Wang X, Li F, Dong Y, Wang J, Chen H, Yuan Z (2019) A second course of stereotactic image-guided robotic radiosurgery for patients with cerebral metastasis. World Neurosurg 123:e621–e628. https://doi.org/10.1016/j.wneu.2018.11.238
Kowalchuk RO, Niranjan A, Lee CC, Yang HC, Liscak R, Guseynova K, Tripathi M, Kumar N, Peker S, Samanci Y, Hess J, Chiang V, Iorio-Morin C, Mathieu D, Pikis S, Wei Z, Lunsford LD, Trifiletti DM, Sheehan JP (2022) Reirradiation with Stereotactic Radiosurgery after local or marginal recurrence of Brain Metastases from previous Radiosurgery. Int J Radiat Oncol Biol Phys 112(3):726–734. https://doi.org/10.1016/j.ijrobp.2021.10.008
Bhatia R, George J, Siu C, Baker BR, Lee EE, Redmond KJ, Jackson C, Bettegowda C, Lim M, Kleinberg LR (2022) Outcomes of Brain Metastases Managed with Resection and Aggressive Reirradiation after Initial Radiosurgery Failure. International Journal of Radiation Oncology, Biology, Physics, Volume 114, Issue 3, Supplement, Pages e48-e49. ISSN 0360–3016. https://doi.org/10.1016/j.ijrobp.2022.07.781
Sneed PK, Chan JW, Ma L, Braunstein SE, Theodosopoulos PV, Fogh SE, Nakamura JL, Boreta L, Raleigh DR, Ziemer BP, Morin O, Hervey-Jumper SL, McDermott MW (2022) Adverse radiation effect and freedom from progression following repeat stereotactic radiosurgery for brain metastases. J Neurosurg 138(1):104–112. https://doi.org/10.3171/2022.4.JNS212597
Loi M, Caini S, Scoccianti S, Bonomo P, De Vries K, Francolini G, Simontacchi G, Greto D, Desideri I, Meattini I, Nuyttens J, Livi L (2020) Stereotactic reirradiation for local failure of brain metastases following previous radiosurgery: systematic review and meta-analysis. Crit Rev Oncol/Hematol 153:103043. https://doi.org/10.1016/j.critrevonc.2020.103043
Bakst RL, Dunkel IJ, Gilheeney S, Khakoo Y, Becher O, Souweidane MM, Wolden SL (2011) Reirradiation for recurrent medulloblastoma. Cancer 117(21):4977–4982. https://doi.org/10.1002/cncr.26148
Wetmore C, Herington D, Lin T, Onar-Thomas A, Gajjar A, Merchant TE, Wetmore C, Herington D, Lin T, Onar-Thomas A, Gajjar A, Merchant TE (2014) Reirradiation of recurrent medulloblastoma: does clinical benefit outweigh risk for toxicity? Cancer 120(23):3731–3737. https://doi.org/10.1002/cncr.28907
Gupta T, Maitre M, Sastri GJ, Krishnatry R, Shirsat N, Epari S et al (2019) Outcomes of salvage re-irradiation in recurrent medulloblastoma correlate with age at initial diagnosis, primary risk-stratification, and molecular subgrouping. J Neurooncol 144(2):283–291. https://doi.org/10.10
Tsang DS, Sarhan N, Ramaswamy V, Nobre L, Yee R, Taylor MD et al (2019) Re-irradiation for children with recurrent medulloblastoma in Toronto, Canada: a 20-year experience. J Neurooncol 145(1):107–114. https://doi.org/10.1007/s11060-019-03272-2
Baroni LV, Freytes C, Fernandez Ponce N, Oller A, Pinto N, Gonzalez A, Maldonado FR, Sampor C, Rugilo C, Lubieniecki F, Alderete D (2021) Craniospinal irradiation as part of re-irradiation for children with recurrent medulloblastoma. J Neurooncol 155(1):53–61. https://doi.org/10.1007/s11060-021-03842-3
Ramaswamy V, Remke M, Bouffet E, Faria CC et al (2013) Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol 14(12):1200–1207. https://doi.org/10.1016/S1470-2045(13)70449-2
Verma J, McCutcheon IE, Waguespack SG, Mahajan A (2014) Feasibility and outcome of re-irradiation in the treatment of multiply recurrent pituitary adenomas. Pituitary 17(6):539–545. https://doi.org/10.1007/s11102-013-0541-x
Minniti G, Paolini S, Rea MLJ, Isidori A, Scaringi C, Russo I, Osti MF, Cavallo L, Esposito V (2013) Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol 111(2):187–194. https://doi.org/10.1007/s11060-012-0999-9
El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S, Bougatf N, Jäkel O, Debus J, Combs SE (2018) Evaluation of particle radiotherapy for the re-irradiation of recurrent intracranial meningioma. Radiation Oncol (London England) 13(1):86. https://doi.org/10.1186/s13014-018-1026-x
Kim M, Lee DH, Kim Rn HJ, Cho YH, Kim JH, Kwon DH (2017) Analysis of the results of recurrent intracranial meningiomas treated with re-radiosurgery. Clin Neurol Neurosurg 153:93–101. https://doi.org/10.1016/j.clineuro.2016.12.014
Weber DC, Bizzocchi N, Bolsi A, Jenkinson MD (2020) Proton Therapy for Intracranial Meningioma for the treatment of Primary/Recurrent Disease Including Re-Irradiation. Front Oncol 10:558845. https://doi.org/10.3389/fonc.2020.558845
Funding
The authors declare no funds, grants, or other support were received during the preparation of this manuscript.
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
GM, RDP, LZ, FM, PT, FDF contributed to the study conception and design. Material preparation, data collection and analysis were performed by GM, RDP and LZ. The first draft of the manuscript and the tables were prepared by GM, RDP and LZ. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Pietro, R., Zaccaro, L., Marampon, F. et al. The evolving role of reirradiation in the management of recurrent brain tumors. J Neurooncol 164, 271–286 (2023). https://doi.org/10.1007/s11060-023-04407-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04407-2