Abstract
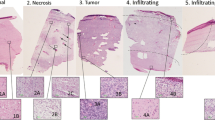
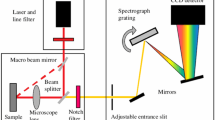
In neurosurgical applications, a tool capable of distinguishing grey matter, white matter, and areas of tumor and/or necrosis in near-real time could greatly aid in tumor resection decision making. Raman spectroscopy is a non-destructive spectroscopic technique which provides molecular information about the tissue under examination based on the vibrational properties of the constituent molecules. With careful measurement and data processing, a spatial step and repeat acquisition of Raman spectra can be used to create Raman images. Forty frozen brain tissue sections were imaged in their entirety using a 300-µm-square measurement grid, and two or more regions of interest within each tissue were also imaged using a 25 µm-square step size. Molecular correlates for histologic features of interest were identified within the Raman spectra, and novel imaging algorithms were developed to compare molecular features across multiple tissues. In previous work, the relative concentration of individual biomolecules was imaged. Here, the relative concentrations of 1004, 1300:1344, and 1660 cm−1, which correspond primarily to protein and lipid content, were simultaneously imaged across all tissues. This provided simple interpretation of boundaries between grey matter, white matter, and diseased tissue, and corresponded with findings from adjacent hematoxylin and eosin-stained sections. This novel, yet simple, multi-channel imaging technique allows clinically-relevant resolution with straightforward molecular interpretation of Raman images not possible by imaging any single peak. This method can be applied to either surgical or laboratory tools for rapid, non-destructive imaging of grey and white matter.



Similar content being viewed by others
References
Bodily L, Mintz AH, Engh J (2013) Combined awake Craniotomy with endoscopic port surgery for resection of a deep-seated temporal lobe glioma: a case report. Case Rep Med 2013:401359
Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA, Grossman RG, Roberts DW (1994) Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery 34:567–576
Johns M, Giller C, Liu H (1998) Computational and in vivo investigation of optical reflectance from human brain to assist neurosurgery. J Biomed Opt 3:437–445
Kalkanis SN, Kast RE, Rosenblum ML, Mikkelsen T, Yurgelevic SM, Nelson KM, Raghunathan A, Poisson LM, Auner GW (2014) Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J Neuro-Oncol 116:477–485
Kast R, Auner G, Rosenblum M, Mikkelsen T, Yurgelevic S, Raghunathan A, Poisson L, Kalkanis S (2014) Raman molecular imaging of brain frozen tissue sections. J Neuro-Oncol 120:55–62
Sterenborg H, Van Gemert M, Kamphorst W, Wolbers J, Hogervorst W (1989) The spectral dependence of the optical properties of human brain. Lasers Med Sci 4:221–227
van der Zee P, Essenpreis M, Delpy DT (1993) Optical properties of brain tissue. In: OE/LASE ‘93: optics, electro-optics, & laser applications in science & engineering, pp 454–465
Muller P, Wilson B (1986) An update on the penetration depth of 630 nm light in normal and malignant human brain tissue in vivo. Phys Med Biol 31:1295
Gebhart S, Lin W, Mahadevan-Jansen A (2006) In vitro determination of normal and neoplastic human brain tissue optical properties using inverse adding-doubling. Phys Med Biol 51:2011
Siebert R, Vu Thi MH, Jean F, Charon Y, Collado-Hilly M, Duval MA, Mandat T, Menard L, Palfi S, Tordjmann T (2008) Development of a new autofluorescence probe for the analysis of normal and tumour brain tissues, pp 699122-699122-699111
Choo LP, Mansfield JR, Pizzi N, Somorjai RL, Jackson M, Halliday WC, Mantsch HH (1995) Infrared spectra of human central nervous system tissue: diagnosis of Alzheimer’s disease by multivariate analyses. Biospectroscopy 1:141–148
Beljebbar A, Dukic S, Amharref N, Manfait M (2010) Ex vivo and in vivo diagnosis of C6 glioblastoma development by Raman spectroscopy coupled to a microprobe. Anal Bioanal Chem 398:477–487
Mizuno A, Hayashi T, Tashibu K, Maraishi S, Kawauchi K, Ozaki Y (1992) Near-infrared FT-Raman spectra of the rat brain tissues. Neurosci Lett 141:47–52
Mizuno A, Kitajima H, Kawauchi K, Muraishi S, Ozaki Y (1994) Near-infrared Fourier transform Raman spectroscopic study of human brain tissues and tumours. J Raman Spectrosc 25:25–29
Ong CW, Shen ZX, He Y, Lee T, Tang SH (1999) Raman microspectroscopy of the brain tissues in the substantia nigra and MPTP-induced Parkinson’s disease. J Raman Spectrosc 30:91–96
Amharref N, Beljebbar A, Dukic S, Venteo L, Schneider L, Pluot M, Manfait M (2007) Discriminating healthy from tumor and necrosis tissue in rat brain tissue samples by Raman spectral imaging. Biochim Biophys Acta (BBA) Biomembranes 1768:2605–2615
Koljenovic S, Schut T, Vincent A, Kros J, Puppels G (2005) Detection of meningioma in dura mater by Raman spectroscopy. Anal Chem 77:7958–7965
Koljenovic S, Choo-Smith L, Bakker Schut T, Kros J, van den Berge H, Puppels G (2002) Discriminating vital tumor from necrotic tissue in human glioblastoma tissue samples by Raman spectroscopy. Lab Invest 82:1265–1277
Krafft C, Sobottka SB, Schackert G, Salzer R (2005) Near infrared Raman spectroscopic mapping of native brain tissue and intracranial tumors. Analyst 130:1070–1077
Krafft C, Sobottka SB, Schackert G, Salzer R (2006) Raman and infrared spectroscopic mapping of human primary intracranial tumors: a comparative study. J Raman Spectrosc 37:367–375
Krafft C, Kirsch M, Beleites C, Schackert G, Salzer R (2007) Methodology for fiber-optic Raman mapping and FTIR imaging of metastases in mouse brains. Anal Bioanal Chem 389:1122–1142
Kirsch M, Schackert G, Salzer R, Krafft C (2010) Raman spectroscopic imaging for in vivo detection of cerebral brain metastases. Anal Bioanal Chem 398:1707–1713
Bergner N, Bocklitz T, Romeike BFM, Reichart R, Kalff R, Krafft C, Popp J (2012) Identification of primary tumors of brain metastases by Raman imaging and support vector machines. Chemom Intell Lab Syst 117:224–232
Feudiger CW, Pfannl R, Orringer DA, Saar BG, Ji M, Zeng Q, Ottoboni L, Ying W, Waeber C, Sims JR (2012) Multicolored stain-free histopathology with coherent Raman imaging. Lab Invest 92:1492–1502
Ji M, Orringer DA, Freudiger CW, Ramkissoon S, Liu X, Lau D, Golby AJ, Norton I, Hayashi M, Agar NY (2013) Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci Transl Med 5:201ra119–201ra119
Morell P, Quarles R et al (1999) Characteristic composition of myelin. In: Siegel GJ, Albers RW (eds) Basic neurochemistry: molecular, cellular and medical aspects, 6th edn. Lippincott-Raven, Philadelphia
Phillips GR, Harris JM (1990) Polynomial filters for data sets with outlying or missing observations: application to charge-coupled-device-detected Raman spectra contaminated by cosmic rays. Anal Chem 62:2351–2357
Mazet V, Carteret C, Brie D, Idier J, Humbert B (2005) Background removal from spectra by designing and minimising a non-quadratic cost function. Chemometrics Intell Lab Syst 76:121–133
Renishaw (2014) Raman images faster than ever before. Renishaw plc. http://www.renishaw.com/media/doc/en/49122d381db649c5925b7fbdfac16d52.docx
Acknowledgments
This work was partially funded by the Hermelin Brain Tumor Center and the Department of Neurosurgery at Henry Ford Hospital, the Smart Sensors and Integrated Microsystems Program at Wayne State University, and the Paul U. Strauss/TEAMS endowed chair position at Wayne State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2015_1929_MOESM1_ESM.tif
Figure S1: Histology and Raman images for the 8 tissues imagedwhich were identified as normal grey and/or white matter on histologic review
11060_2015_1929_MOESM2_ESM.tif
Figure S2: Histology and Raman images for the 8 tissues which showed a boundary between normal tissue and pathologic tissue on H&E review emphasize the relative improvement in distinguishing tissue boundaries.
Rights and permissions
About this article
Cite this article
Kast, R., Auner, G., Yurgelevic, S. et al. Identification of regions of normal grey matter and white matter from pathologic glioblastoma and necrosis in frozen sections using Raman imaging. J Neurooncol 125, 287–295 (2015). https://doi.org/10.1007/s11060-015-1929-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1929-4