Abstract
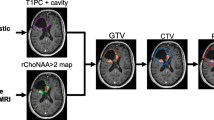
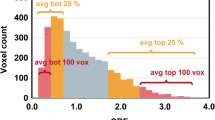
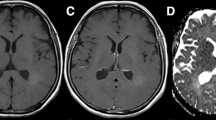
We observed a stripe-like pattern of regional cerebral blood volume (rCBV) increase in a defined region adjacent to the contrast enhancement (CE) on MRI of glioblastomas (GBM) that we defined as the “striate sign” (SS). We hypothesized that the SS marks infiltration of GBM outside the CE volume transforming into future CE tumor in the follow-up. T2*-weighted dynamic susceptibility-weighted CE (DSC)-MRI, and T1 and T2-weighted images (WI) of 16 patients with GBM were retrospectively evaluated in a baseline MRI performed before neurosurgery. In seven of these patients we also performed a 1H MR spectroscopic imaging (1H MRSI). The regions of interest (ROI) delineating the SS were defined on rCBV maps for each patient. ROIs were overlaid on follow-up T1-WI and T2-WI MRI performed 3, 6, and 9 months after neurosurgery. Size and maximum signal intensity (max SI) of de novo CE within the area of the SS were analyzed. Statistical analysis was performed with the Friedman test (P < 0.05). In 15/16 patients de novo CE completely covered the area of the SS within nine months. Normalized max SI of de-novo CE of the 3, 6, and 9-months follow-up MR examinations were significantly higher than in the baseline MRI (P < 0.001). Normalized choline was increased within the SS in all patients with de novo CE (n = 6). De-novo CE appeared within the SS in all patients (96% of all slices). This implies that the SS might indicate the site of future CE tumor, which represents the area of tumor growth after neurosurgery.





Similar content being viewed by others
References
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66:865–874
Silbergeld D, Madsen C, Chicoine M (1995) Isolation of human malignant glioma cells from histologically normal brain. Presented at the CNS annual meeting, San Francisco
Burger PC, Dubois PJ, Schold SC Jr, Smith KR Jr, Odom GL, Crafts DC, Giangaspero F (1983) Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg 58:159–169
Gaspar LE, Fisher BJ, Macdonald DR, LeBer DV, Halperin EC, Schold SC Jr, Cairncross JG (1992) Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys 24:55–57
Chiang IC, Kuo YT, Lu CY, Yeung KW, Lin WC, Sheu FO, Liu GC (2004) Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46:619–627
Sadeghi N, Salmon I, Decaestecker C, Levivier M, Metens T, Wikler D, Denolin V, Rorive S, Massager N, Baleriaux D, Goldman S (2007) Stereotactic comparison among cerebral blood volume, methionine uptake, and histopathology in brain glioma. Am J Neuroradiol 28:455–461
Stadlbauer A, Moser E, Gruber S, Buslei R, Nimsky C, Fahlbusch R, Ganslandt O (2004) Improved delineation of brain tumors. An automated method for segmentation based on pathologic changes of 1H-MRSI metabolites in gliomas. Neuroimage 23:454–461
Hattingen E, Raab P, Franz K, Zanella FE, Lanfermann H, Pilatus U (2008) Myo-inositol: a marker of reactive astrogliosis in glial tumors? NMR Biomed 21:233–241
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Hattingen E, Pilatus U, Franz K, Zanella FE, Lanfermann H (2007) Evaluation of optimal echo time for 1 H-spectroscopic imaging of brain tumors at 3 Tesla. J Magn Reson Imaging 26:427–431
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part i: mathematical approach and statistical analysis. Magn Reson Med 36:715–725
Rosen BR, Belliveau JW, Vevea JM, Brady TJ (1990) Perfusion imaging with NMR contrast agents. Magn Reson Med 14:249–265
Sorensen AG, Copen WA, Ostergaard L, Buonanno FS, Gonzalez RG, Rordorf G, Rosen BR, Schwamm LH, Weisskoff RM, Koroshetz WJ (1999) Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology 210:519–527
Cha S, Tihan T, Crawford F, Fischbein NJ, Chang S, Bollen A, Nelson SJ, Prados M, Berger MS, Dillon WP (2005) Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. Am J Neuroradiol 26:266–273
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. Am J Neuroradiol 24:1989–1998
Wetzel SG, Cha S, Johnson G, Lee P, Law M, Kasow DL, Pierce SD, Xue X (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224:797–803
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Sehgal V, Delproposto Z, Haddar D, Haacke EM, Sloan AE, Zamorano LJ, Barger G, Hu J, Xu Y, Prabhakaran KP, Elangovan IR, Neelavalli J, Reichenbach JR (2006) Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging 24:41–51
Lupo JM, Cha S, Chang SM, Nelson SJ (2007) Analysis of metabolic indices in regions of abnormal perfusion in patients with high-grade glioma. Am J Neuroradiol 28:1455–1461
Chamberlain MC, Chalmers L (2007) A pilot study of primary temozolomide chemotherapy and deferred radiotherapy in elderly patients with glioblastoma. J Neurooncol 82:207–209
Galanaud D, Chinot O, Nicoli F, Confort-Gouny S, Le Fur Y, Barrie-Attarian M, Ranjeva JP, Fuentes S, Viout P, Figarella-Branger D, Cozzone PJ (2003) Use of proton magnetic resonance spectroscopy of the brain to differentiate gliomatosis cerebri from low-grade glioma. J Neurosurg 98:269–276
Scherer H (1940) The forms of growth in gliomas and their practical significance. Brain 63:1–35
Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P (2006) Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia 53:799–808
Bolteus AJ, Berens ME, Pilkington GJ (2001) Migration and invasion in brain neoplasms. Curr Neurol Neurosci Rep 1:225–232
Giese A, Bjerkvig R, Berens ME (2003) Westphal M: cost of migration: Invasion of malignant gliomas and implications for treatment. J Clin Oncol 21:1624–1636
Ljubimova JY, Lakhter AJ, Loksh A, Yong WH, Riedinger MS, Miner JH, Sorokin LM, Ljubimov AV, Black KL (2001) Overexpression of alpha 4 chain-containing laminins in human glial tumors identified by gene microarray analysis. Cancer Res 61:5601–5610
Aronen HJ, Perkio J (2002) Dynamic susceptibility contrast MRI of gliomas. Neuroimaging Clin N Am 12:501–523
Provenzale JM, Mukundan S, Barboriak DP (2006) Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology 239:632–649
Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D (2002) Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology 223:11–29
Lev MH, Rosen BR (1999) Clinical applications of intracranial perfusion MR imaging. Neuroimaging Clin N Am 9:309–331
Preul C, Kuhn B, Lang EW, Mehdorn HM, Heller M, Link J (2003) Differentiation of cerebral tumors using multi-section echo planar MR perfusion imaging. Eur J Radiol 48:244–251
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. Am J Neuroradiol 27:859–867
Principi M, Italiani M, Guiducci A, Aprile I, Muti M, Giulianelli G, Ottaviano P (2003) Perfusion MRI in the evaluation of the relationship between tumour growth, necrosis and angiogenesis in glioblastomas and grade 1 meningiomas. Neuroradiology 45:205–211
Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, Shigematsu Y, Liang L, Ge Y, Ushio Y, Takahashi M (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. Am J Roentgenol 171:1479–1486
Acknowledgments
The authors would like to thank Dr Alina Jurcoane for carefully reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blasel, S., Franz, K., Ackermann, H. et al. Stripe-like increase of rCBV beyond the visible border of glioblastomas: site of tumor infiltration growing after neurosurgery. J Neurooncol 103, 575–584 (2011). https://doi.org/10.1007/s11060-010-0421-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0421-4