Abstract
Background and purpose
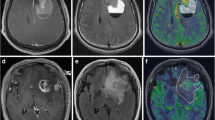
Gliomatosis cerebri (GC) is a rare growth pattern of glioblastoma whose diffuse nature is reflected by unspecific, relatively uniform findings on conventional MRI. In the present study we sought to evaluate the additional value of diffusion (DWI) and perfusion weighted (PWI) MRI for a more detailed characterization.
Methods
We analyzed the MRI findings in patients with histologically proven glioblastoma with GC growth pattern with a specific emphasis on T2 lesion pattern, volume, relative apparent diffusion coefficient (rACD), and relative cerebral blood volume (rCBV) and compared these to age-/gender-matched patients with localized glioblastoma.
Results
Overall, 16 patients (median age 59.5 years, 4 male) were included in the study. Of these, 8 patients had a glioblastoma with GC growth pattern, and 8 a classical localized growth pattern. While the median rADC (1.27 [IQR 1.12–1.41]) within the T2 lesion was significant lower in glioblastoma with GC growth pattern compared to localized glioblastoma (1.74 [IQR 1.45–1.96]; p = 0.003), the median T2 lesion volume and rCBV within the T2 lesion did not differ significantly. Furthermore, six patients with glioblastoma with GC growth pattern showed focal areas with significantly reduced rADC (p = 0.043), and/or increased rCBV (p = 0.028).
Conclusions
Lower rADC in glioblastoma with GC growth pattern might reflect the diffuse tumor cell infiltration whereas focal areas with decreased rADC and/or increased rCBV probably indicate high tumor cell density and/or abnormal tumor vessels which may be useful for biopsy guidance.


Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Scherer J (1940) The forms of growth in gliomas and their practical significance. Brain 40:631–635
Holland EC (2000) Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA 97(12):6242–6244
Thomas RP, Xu LW, Lober RM, Li G, Nagpal S (2013) The incidence and significance of multiple lesions in glioblastoma. J Neurooncol 112(1):91–97
Wesseling P, Capper D (2018) WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol 44(2):139–150
Carpio-O’Donovan R, Korah I, Salazar A, Melancon D (1996) Gliomatosis cerebri. Radiology 198(3):831–835
Chen S, Tanaka S, Giannini C, Morris J, Yan ES, Buckner J, Lachance DH, Parney IF (2013) Gliomatosis cerebri: clinical characteristics, management, and outcomes. J Neurooncol 112(2):267–275
Barajas RF Jr, Hodgson JG, Chang JS, Vandenberg SR, Yeh RF, Parsa AT, McDermott MW, Berger MS, Dillon WP, Cha S (2010) Glioblastoma multiforme regional genetic and cellular expression patterns: influence on anatomic and physiologic MR imaging. Radiology 254(2):564–576
Barajas RF Jr, Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, Parsa AT, Aghi MK, McDermott MW, Berger MS, Cha S, Chang SM, Nelson SJ (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncology 14(7):942–954
Peretti-Viton P, Brunel H, Chinot O, Daniel C, Barrie M, Bouvier C, Figarella-Branger D, Fuentes S, Dufour H, Grisoli F (2002) Histological and MR correlations in Gliomatosis cerebri. J Neurooncol 59(3):249–259
Gempt J, Soehngen E, Forster S, Ryang YM, Schlegel J, Zimmer C, Meyer B, Ringel F, Grams AE, Forschler A (2014) Multimodal imaging in cerebral gliomas and its neuropathological correlation. Eur J Radiol 83(5):829–834
Yu A, Li K, Li H (2006) Value of diagnosis and differential diagnosis of MRI and MR spectroscopy in gliomatosis cerebri. Eur J Radiol 59(2):216–221
Desclee P, Rommel D, Hernalsteen D, Godfraind C, de Coene B, Cosnard G (2010) Gliomatosis cerebri, imaging findings of 12 cases. J Neuroradiol 37(3):148–158
Constans JM, Collet S, Kauffmann F, Hossu G, Dou W, Ruan S, Rioult F, Derlon JM, Lechapt-Zalcmann E, Chapon F, Valable S, Theron J, Guillamo JS, Courtheoux P (2011) Five-year longitudinal MRI follow-up and (1)H single voxel MRS in 14 patients with gliomatosis treated with temodal, radiotherapy and antiangiogenic therapy. Neuroradiol J 24(3):401–414
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27(4):859–867
Tatu L, Moulin T, Bogousslavsky J, Duvernoy H (1996) Arterial territories of human brain: brainstem and cerebellum. Neurology 47(5):1125–1135
Tatu L, Moulin T, Bogousslavsky J, Duvernoy H (1998) Arterial territories of the human brain: cerebral hemispheres. Neurology 50(6):1699–1708
Rorden C, Karnath HO, Bonilha L (2007) Improving lesion-symptom mapping. J Cogn Neurosci 19(7):1081–1088
Stummer W (2007) Mechanisms of tumor-related brain edema. Neurosurg Focus 22(5):E8
Gupta A, Young RJ, Karimi S, Sood S, Zhang Z, Mo Q, Gutin PH, Holodny AI, Lassman AB (2011) Isolated diffusion restriction precedes the development of enhancing tumor in a subset of patients with glioblastoma. AJNR Am J Neuroradiol 32(7):1301–1306
Yang S, Wetzel S, Law M, Zagzag D, Cha S (2002) Dynamic contrast-enhanced T2*-weighted MR imaging of gliomatosis cerebri. AJNR Am J Neuroradiol 23(3):350–355
Rizzo L, Crasto SG, Moruno PG, Cassoni P, Ruda R, Boccaletti R, Brosio M, De Lucchi R, Fava C (2009) Role of diffusion- and perfusion-weighted MR imaging for brain tumour characterisation. Radiol Med 114(4):645–659
Hu LS, Eschbacher JM, Dueck AC, Heiserman JE, Liu S, Karis JP, Smith KA, Shapiro WR, Pinnaduwage DS, Coons SW, Nakaji P, Debbins J, Feuerstein BG, Baxter LC (2012) Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol 33(1):69–76
Wang B, Zhao P, Zhang Y, Ge M, Lan C, Li C, Pang Q, Xu S, Liu Y (2018) Quantitative dynamic susceptibility contrast perfusion-weighted imaging-guided customized gamma knife re-irradiation of recurrent high-grade gliomas. J Neurooncol 139(1):185–193
Chiang IC, Kuo YT, Lu CY, Yeung KW, Lin WC, Sheu FO, Liu GC (2004) Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46(8):619–627
Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran VM, Cotton F (2006) Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology 48(3):150–159
Tsougos I, Svolos P, Kousi E, Fountas K, Theodorou K, Fezoulidis I, Kapsalaki E (2012) Differentiation of glioblastoma multiforme from metastatic brain tumor using proton magnetic resonance spectroscopy, diffusion and perfusion metrics at 3 T. Cancer Imaging 12:423–436
Neska-Matuszewska M, Bladowska J, Sasiadek M, Zimny A (2018) Differentiation of glioblastoma multiforme, metastases and primary central nervous system lymphomas using multiparametric perfusion and diffusion MR imaging of a tumor core and a peritumoral zone-searching for a practical approach. PLoS ONE 13(1):e0191341
Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW (2002) High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 222(3):715–721
Lehmann P, Saliou G, de Marco G, Monet P, Souraya SE, Bruniau A, Vallee JN, Ducreux D (2012) Cerebral peritumoral oedema study: does a single dynamic MR sequence assessing perfusion and permeability can help to differentiate glioblastoma from metastasis? Eur J Radiol 81(3):522–527
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alex Förster: none. Stefanie Brehmer received travel support from Carl Zeiss Meditec AG. Marcel Seiz-Rosenhagen: none. Iris Mildenberger: none. Frank A. Giordano serves as consultant and speaker for Carl Zeiss Meditec AG, NOXXON Pharma AG, Merck Serono GmbH, Roche Pharma AG, Siemens Healthcare Diagnostics GmbH, and holds patents related with Carl Zeiss Meditec AG. Holger Wenz: none. David Reuss: none. Daniel Hänggi: none. Christoph Groden: none.
Ethical approval
This study has been approved by the local institutional review board (Medizinische Ethikkommission II der Medizinischen Fakultät Mannheim) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Patient consent was waived for this analysis by the local institutional review board due to its retrospective nature.
Rights and permissions
About this article
Cite this article
Förster, A., Brehmer, S., Seiz-Rosenhagen, M. et al. Heterogeneity of glioblastoma with gliomatosis cerebri growth pattern on diffusion and perfusion MRI. J Neurooncol 142, 103–109 (2019). https://doi.org/10.1007/s11060-018-03068-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03068-w