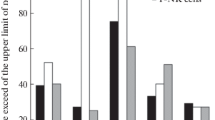
A comparative analysis of the contents of CD4+, CD8+, and CD16/32+ cells in the peripheral blood and spleen of intact (non-immunized) ASC mice with a genetically determined depression-like state and mice of the parental CBA strain, without depressive behavior, is presented. ASC mice showed decreases in the relative number of CD16/32+ and CD4+ cells, accumulation of CD8+ cells, and a decrease in the index of immunoreactivity (CD4/CD8). Changes in the subpopulations of study cells in intact ASC mice were accompanied by reduced responses of animals to a T-dependent antigen – sheep erythrocytes (5·108). There were significant reductions in the relative and absolute numbers of IgM-antibody-forming cells (AFC) on post-immunization days 4 and 5 and IgG AFC on day 6 in the spleens of ASC mice, as compared with CBA mice. The possible mechanisms of reductions in immunoreactivity in the genetically determined depression-like state are discussed.
Similar content being viewed by others
References
E. L. Al’perina, A. V. Kulikov, N. K. Popova, and G. V. Idova, “Nature of the immune response in ASC (antidepressant-sensitive catalepsy) mice,” Byull. Eksperim. Biol. Med., 144, No. 8, 188–190 (2007).
E. B. Arushanyan and E. B. Beier, “Relationship between psychoemotional status and the immune system,” Usp. Fiziol. Nauk., 35, No. 4, 49–64 (2004).
D. V. Gazovkina, A. V. Kulikov, E. M. Kondaurova, and N. K. Popova, “Breeding for predisposition to catalepsy increases depression-like behavior in mice,” Genetika, 41, No. 9, 122–1228 (2005).
L. V. Devoino, G. V. Idova, and E. L. Al’perina, Pyschoneuroimmunomodulation. Behavior and Immunity [in Russian], Nauka, Novosibirsk (2009).
L. V. Devoino and R. Yu. Il’yuchenok, Neurotransmitter Systems in Psychoneuroimmunomodulation: Serotonin, Dopamine, GABA, Neuropeptides [in Russian], TsERIS, Novosibirsk (1993).
N. I. Dubrovina, D. R. Zinov’ev, D. V. Zinov’eva, and A. V. Kulikov, “Learning and extinction of a passive avoidance reaction in mice with high predisposition to catalepsy,” Ros. Fiziol. Zh. im. I. M. Sechenova, 94, No. 6, 609–616 (2008).
G. V. Idova, M. A. Cheido, E. N. Zhukova, et al., “Effects of the type 1-A serotonin receptor agonist 8-OH-DPAT on the immune response,” Byull. Eksperim. Biol. Med., 132, No. 10, 432–434 (2001).
G. V. Idova, T. A. Pavina, E. L. Al’perina, and L. V. Devoino, “Effects of submissive and aggressive types of behavior on changes in the numbers of CD4+ and CD8+ T lymphocytes in bone marrow,” Immunologiya, 1, 24–26, 00
E. M. Kondaurova, D. V. Bazovkina, and A. V. Kulikov, “Studies of the interaction of catalepsy with anxiety, aggression, and depression-like behavior using congenic mouse strains,” Ros. Fiziol. Zh. im. I. M. Sechenova, 96, No. 5, 464–471 (2010).
A. V. Kulikov, V. S. Naumenko, D. V. Bazovkina, et al., “Effects of the terminal fragment of mouse chromosome 13 on the predisposition to catalepsy and the expression of genes encoding tryptophan hydroxylase 2, the serotonin transporter, and 5-HT1A receptors in the brain,” Byull. Eksperim. Biol. Med., 147, 553–556 (2009).
G. N. Kryzhanovskii, I. G. Akmaev, S. V. Magaeva, and S. G. Morozov, Neuroimmunoendocrine Interactions in Health and Disease [in Russian], Med. Kniga, Moscow (2010).
V. Ya. Semke, T. P. Vetlugina, T. I. Nevidimova, et al., Clinical Neuroimmunopathology [in Russian], RASKO, Tomsk (2003).
M. V. Tenditnik, A. V. Shurlygina, E. V. Mel’nikova, et al., “Changes in the subpopulation composition of lymphocytes in the immunocompetent organs of mice in conditions of chronic social stress,” Ros. Fiziol. Zh. im. I. M. Sechenova, 90, No. 12, 1522–1529 (2004).
L. Capuron, A. Miller, and M. R. Irwin, “Psychoneuroimmunology of depressive disorder: mechanisms and clinical implications,” Psychoneuroimmunol., 1, 509–530 (2007).
B. W. Dunlop and C. B. Nemeroff, “The role of dopamine in pathophysiology of depression,” Arch. Gen. Psychiatry, 64, No. 3, 327–337 (2007).
H. Enger, M. T. Bailey, A. Engler, and J. F. Sheridan, “Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen,” J. Neuroimmunol., 148, No. 1–2, 106–115 (2004).
J. Hennig, H. Becker, and P. Netter, “5-HT agonist-induced changes in peripheral immune cells in healthy volunteers: the impact of personality,” Behav. Brain Res., 73, No. 1–2, 359–363 (1995).
G. V. Idova and S. M. Davydova, “Involvement of presynaptic 5-HT1A receptors in immunomodulation in conditions of psychoemotional tension,” Neurosci. Behav. Physiol., 40, No. 5, 495–499 (2010).
M. R. Irwin and A. H. Miller, “Depressive disorders and immunity: 20 years of progress and discovery,” Brain Behav. Immun., 21, No. 4, 374–383 (2007).
G. S. Ladics, “Primary immune response to sheep red blood cells (SRBS) as the conventional T-cell dependent antibody response (TDAR) test,” J. Immunotoxicol., 4, No. 2, 149–152 (2007).
C. A. Ottaway and A. J. Husband, “The influence of neuroendocrine pathways on lymphocyte migration,” Immunol. Today, 5, No. 1, 511–517 (1994).
D. H. Overstreet, R. C. Commissaris, R. De La Garza, et al., “Involvement of 5-HT1A receptors in animal tests of anxiety and depression: evidence from genetic models,” Stress, 6, No. 2, 101–110 (2003).
J. M. Petitto, “Behavioral genetics and immunity,” in: Psychoneuroimmunology, Elsevier Acad. Press, San Diego (2001), Vol. 2, pp. 173–186.
M. A. Tikhonova, E. L. Alperina, T. G. Tolstikova, et al., “Effects of chronic fluoxetine treatment on catalepsy and the immune response in mice with a genetic predisposition to freezing reactions: the roles of types 1A and 2A serotonin receptors and the tph2 and SERT genes,” Neurosci. Behav. Physiol., 40, No. 5, 521–527 (2010).
J. Veenstra-Vender Weele, G. M. Anderson, and E. H. Cook, Jr., “Pharmacogenetics and the serotonin system: initial studies and future direction,” Eur. J. Pharmacol., 410, No. 2–3, 165–181 (2000).
M. E. I. Yacoubi and J. M. Vaugeois, “Genetic rodent models of depression,” Curr. Opin. Pharmacol., 7, No. 1, 3–7 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 98, No. 2, pp. 194–201, February, 2012.
Rights and permissions
About this article
Cite this article
Idova, G.V., Al’perina, E.L., Gevorgyan, M.M. et al. T-Lymphocyte Subpopulation Composition and the Immune Response in Depression-Like Behavior in ASC Mice. Neurosci Behav Physi 43, 946–950 (2013). https://doi.org/10.1007/s11055-013-9833-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-013-9833-x