Abstract
This systematic review presents an overview, from 2011 to 2022, of how pure B12N12 and B12N12-modified nanocages have been used to detect toxic gases from studies based on density functional theory (DFT). Searches were carried out in the ISI Web of Science, Science Direct, and Scopus databases to identify all studies related to B12N12 nanocages and, with the application of exclusion and inclusion criteria, to select articles involving theoretical studies of pure B12N12 and B12N12-modified nanocages as toxic gas sensors. In addition, from our analysis of these articles, we infer that a pure B12N12 nanocage can selectively detect O3, HNO, NO, CO, ClCN, and FCN gases. However, it cannot detect N2O and NH3. In contrast, metal-modified B12N12 nanocage can detect a wider range of gases such as CO, NH3, PH3, AsH3, SO2, O3, COCl2, NCCN, and ClCN. However, few studies have discussed the interference effects due to the presence of other gases in the atmosphere and/or the incorporation of different structural modifications (decorated, doped, and encapsulated) into the cage. Lastly, this systematic review will guide the development of new applications of nanocages in the selective detection of toxic gases.
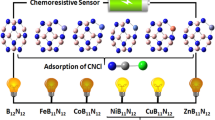
Graphical Abstract
Schematic representation of the detection of different toxic gases on a B12N12 nanocage surface.



Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
References
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: Buckminsterfullerene. Nature 318:162–163. https://doi.org/10.1038/318162a0
Kent PRC, Towler MD, Needs RJ, Rajagopal G (2000) Phys Rev B 62:15394. https://doi.org/10.1103/PhysRevB.62.15394
Lu X, Chen Z (2005) Curved Pi-conjugation, aromaticity, and the related chemistry of small fullerenes (<C60) and single-walled carbon nanotubes. Chem Rev 105:3643–3696. https://doi.org/10.1021/cr030093d
Enyashin AN (2010) Theoretical studies of inorganic fullerenes and fullerene-like nanoparticles. Isr J Chem 50:468–483. https://doi.org/10.1002/ijch.201000058
Kaviani S, Shahab S, Sheikhi M, Potkin V, Zhou H (2021) A DFT study of Se-decorated B12N12 nanocluster as a possible drug delivery system for ciclopirox. Comput Theor Chem 1201:113246. https://doi.org/10.1016/j.comptc.2021.113246
Liao Z, Song G, Yang Z, Ren H (2021) Adsorption and desorption behaviors of hydroxyurea drug on delivery systems of B12N12 fullerene and its Al-, Si- and P-dopings from theoretical perspective. Mol Phys 119:e1921296. https://doi.org/10.1080/00268976.2021.1921296
Rad AS, Ayub K (2016) Enhancement in hydrogen molecule adsorption on B12N12 nano-cluster by decoration of nickel. Int J Hydrog Energy 41:22182–22191. https://doi.org/10.1016/j.ijhydene.2016.08.158
Janjua MRSA (2021) Theoretical framework for encapsulation of inorganic B12N12 nanoclusters with alkaline earth metals for efficient hydrogen adsorption: a step forward toward hydrogen storage materials. Inorg Chem 60:2816–2828. https://doi.org/10.1021/acs.inorgchem.0c03730
Hosseini J, Rastgou A, Moradi R (2017) F-encapsulated B12N12 fullerene as an anode for Li-ion batteries: a theoretical study. J Mol Liq 225:913–918. https://doi.org/10.1016/j.molliq.2016.11.025
Nejati K, Hosseinian A, Bekhradnia A, Vessally E, Edjlali L (2017) Na-ion batteries based on the inorganic BN nanocluster anodes: DFT studies. J Mol Graph Model 74:1–7. https://doi.org/10.1016/j.jmgm.2017.03.001
Alvand R, Rezaei-Sameti M (2021) The H+ ions and static electric field effects on the adsorption and detection of cyanogen fluoride on the surface of boron nitride nanocage: a DFT, TD-DFT study. Adsorption 27:91–104. https://doi.org/10.1007/s10450-020-00278-5
Kalateh K, Abdolmanafi A (2015) Study of B12N12 and AlB11N12 fullerene as H2S absorbent and sensor by computational method. Int J New Chem 2:1–7. https://doi.org/10.22034/IJNC.2015.15629
Rostamoghli R, Vakili M, Banaei A, Pourbashir E, Jalalierad K (2018) Applying the B12N12 nanoparticle as the CO, CO2, H2O and NH3 sensor. Chem Rev Lett 1:31–36. https://doi.org/10.22034/crl.2018.85214
Silva ALP, Silva ACA, Varela Júnior JJG (2022) Putrescine adsorption on pristine and Cu-decorated B12N12 nanocages: a density functional theory study. Comput Theor Chem 1215:113836. https://doi.org/10.1016/j.comptc.2022.113836
Soltani A, Ramezanitaghartapeh M, Javan MB, Baei MT, Lup ANK, Mahon PJ, Aghaei M (2020) Influence of the adsorption of toxic agents on the optical and electronic properties of B12N12 fullerene in the presence and absence of an external electric field. New J Chem 44:14513–14528. https://doi.org/10.1039/D0NJ01868F
Rauf HG, Mahmood EA, Majedi S, Sofi M (2019) Adsorption behavior of the Al- and Ga-doped B12N12 nanocages on COn (n=1, 2) and HnX (n=2, 3 and X=O, N): A comparative study. Chem Rev Lett 2:140–150. https://doi.org/10.22034/crl.2020.214660.1029
Barea E, Montoro C, Navarro JAR (2014) Toxic gas removal – metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem Soc Rev 43:5419–5430. https://doi.org/10.1039/C3CS60475F
Kinoshita H, Türkan H, Vucinic S, Naqvi S, Bedair R, Rezaee R, Tsatsakis A (2020) Carbon Monoxide Poisoning. Toxicol Rep 7:169–173. https://doi.org/10.1016/j.toxrep.2020.01.005
Jensen F, Toftlund H (1993) Structure and stability of C24 and B12N12 isomers. Chem Phys Lett 201:89–96. https://doi.org/10.1016/0009-2614(93)85039-Q
Strout DL (2000) Structure and stability of boron nitrides: isomers of B12N12. J Phys Chem A 104:3364–3366. https://doi.org/10.1021/jp994129a
Seifert G, Fowler RW, Mitchell D, Porezag D, Frauenheim Th (1997) Boron-nitrogen analogues of the fullerenes: electronic and structural properties. Chem Phys Lett 268:352–358. https://doi.org/10.1016/S0009-2614(97)00214-5
Xie W, Wang J, Wang J, Wu X, Wang Z, Zhang R-Q (2020) High-angular-momentum orbitals and superatomic characteristics of boron-nitrogen cages. J Phys Chem C 124:3881–3885. https://doi.org/10.1021/acs.jpcc.9b11351
Oku T, Nishiwaki A (2004) Narita I (2004) Formation and atomic structure of B12N12 nanocage clusters studied by mass spectrometry and cluster calculation. Sci Technol Adv Mater 5:635–645. https://doi.org/10.1016/j.stam.2004.03.017
Zhu Y-C, Bando Y, Yin L-W, Golberg D (2004) Hollow boron nitride (BN) nanocages and BN-nanocage-encapsulated nanocrystals. Chem Eur J 10:3667–3672. https://doi.org/10.1002/chem.200400002
Oku T, Nishiwaki A, Narita I (2004) Formation and structure of B28N28 clusters studied by mass spectrometry and molecular orbital calculation. Solid State Commun 130:171–173. https://doi.org/10.1016/j.ssc.2004.02.004
Oku T, Nishiwaki A, Narita I (2004) Formation and structures of B36N36 and Y@B36N36 clusters studied by high-resolution electron microscopy and mass spectrometry. J Phys Chem Solids 65:369–372. https://doi.org/10.1016/j.jpcs.2003.09.010
Oku T (2015) Hydrogen storage in boron nitride and carbon nanomaterials. Energies 8:319–337. https://doi.org/10.3390/en8010319
Oku T, Kuno M, Kitahara H, Narita I (2001) Formation, atomic structures and properties of boron nitride and carbon nanocage fullerene materials. Int J Inorg Materials. 3:597–612. https://doi.org/10.1016/S1466-6049(01)00169-6
Silva ALP, Silva ACA, Navis CN, Varela Júnior JJG (2021) Theoretical study of putrescine and X12Y12 (X=Al, B and Y=N, P) nanocage interactions. J Nanoparticle Res 23:108. https://doi.org/10.1007/s11051-021-05211-7
Kaviani S, Shahab S, Sheikhi M (2021) Adsorption of alprazolam drug on the B12N12 and Al12N12 nano-cages for biological applications: a DFT study. Physica E Low Dimens Syst Nanostruct 126:114473. https://doi.org/10.1016/j.physe.2020.114473
Shamim SUD, Miah MH, Hossain MR, Hasan MM, Hossain MK, Hossain MA, Ahmed F (2022) Theoretical investigation of emodin conjugated doped B12N12 nanocage by means of DFT, QTAIM and PCM analysis. Physica E Low Dimens Syst Nanostruct 136:115027. https://doi.org/10.1016/j.physe.2021.115027
Zaporotskova IV, Boroznina NP, Parkhomenko YN, Kozhitov LL (2016) Carbon nanotubes: sensor properties. A review Mod Electron Mater 2:95–105. https://doi.org/10.1016/j.moem.2017.02.002
Silva ALP, Varela Júnior JJG (2022) Density functional theory study of Cu-modified B12N12 nanocage as a chemical sensor for carbon monoxide gas. Inorg Chem no prelo. https://doi.org/10.1021/acs.inorgchem.2c01621
Hadipour NL, Peyghan AA, Soleymanabadi H (2015) J Phys Chem C 119:6398–6404. https://doi.org/10.1021/jp513019z
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2011) Toxic CO detection by B12N12 nanocluster. Microelectronics J 42:1400–1403. https://doi.org/10.1016/j.mejo.2011.10.010
Beheshtian J, Peyghan AA, Bagheri Z, Kamfiroozi M (2012) Interaction of small molecules (NO, H2, N2, and CH4) with BN nanocluster surface. Struct Chem 23:1567–1572. https://doi.org/10.1007/s11224-012-9970-9
Beheshtian J, Kamfiroozi M, Bagheri Z, Peyghan AA (2012) B12N12 nano-cage as potential sensor for NO2 detection. Chin J Chem Phys 25:60–64. https://doi.org/10.1088/1674-0068/25/01/60-64
Peng S, Cho K (2003) Nano Lett 3:513–517. https://doi.org/10.1021/nl034064u
Silva LB (2004) Fagan SB Mota R. Nano Lett 4:65–67. https://doi.org/10.1021/nl034873d
Baierle RJ, Schmidt TM, Fazzio A (2007) Solid state Commun 142:49–53. https://doi.org/10.1016/j.ssc.2007.01.036
Wang R, Zhang D (2008) Aust J Chem 61:941–945. https://doi.org/10.1071/CH08226
Vadalkar S, Chodvadiya D, Som NN, Vyas KN, Jha PK, Chakraborty B (2022) ChemistrySelect 7:e202103874. https://doi.org/10.1002/slct.202103874
Peng S, Cho K, Qi P, Dai H (2004) Ab initio study of CNT NO2 gas sensor. Chem Phys Lett 387:271–276. https://doi.org/10.1016/j.cplett.2004.02.026
Onsori S, Liyaghati-Delshad M (2017) The effects of different Minnesota functionals on the sensitivity of boron nitride nanocluster to nitrogen dioxide. Chem Phys Lett 680:22–27. https://doi.org/10.1016/j.cplett.2017.05.030
Vessally E, Soleimani-Amiri S, Hosseinian A, Edjlali L, Bekhradnia A (2017) Physica E Low Dimens Syst Nanostruct 87:308–311. https://doi.org/10.1016/j.physe.2016.11.010
Vahabi V, Soleymanabadi H (2016) A quantum mechanical analysis of the electronic response of bn nanocluster to formaldehyde. J Mex Chem Soc 60:34–39
Shakerzadeh E (2016) A DFT study on the formaldehyde (H2CO and (H2CO)2) monitoring using pristine B12N12 nanocluster. Physica E Low Dimens Syst Nanostruct 78:1–9. https://doi.org/10.1016/j.physe.2015.11.038
Baei MT (2013) B12N12 sodalite like cage as potential sensor for hydrogen cyanide. Comput Theor Chem 1024:28–33. https://doi.org/10.1016/j.comptc.2013.09.018
Solimannejad M, Noormohammadbeigi M (2016) HNO detection by nanosized B12N12 cage: a DFT/TDDFT study. Phys Chem Res 4:693–706. https://doi.org/10.22036/pcr.2016.32637
Panahyab A, Soleymanabadi H (2016) Ozone adsorption on a BN fullerene-like nano-cage: a DFT study. Main Group Met Chem 15:347–354. https://doi.org/10.3233/MGC-160214
Bahrami A, Qarai MB, Hadipour NL (2017) The electronic and structural responses of B12N12 nanocage toward the adsorption of some nonpolar X2 molecules: X = (Li, Be, B, N, O, F, Cl, Br, I): A DFT approach. Comput Theor Chem 1108:63–69. https://doi.org/10.1016/j.comptc.2017.03.018
Vessally E, Behmagham F, Massuomi B, Hosseinian A, Nejati K (2017) Selective detection of cyanogen halides by BN nanocluster: a DFT study. J Mol Model 23:138. https://doi.org/10.1007/s00894-017-3312-1
Badran HM, Eid KhM, Ammar HY (2020) A DFT study on the effect of the external electric field on ammonia interaction with boron nitride nano-cage. J Phys Chem Solids 141:109399. https://doi.org/10.1016/j.jpcs.2020.109399
Silva ALP, Varela Júnior JJG (2022) Carbon monoxide interaction with B12N12 nanocage with and without an external electric field: a DFT study. J Nanopart Res 24:1. https://doi.org/10.1007/s11051-021-05382-3
Peyghan AA, Soleymanabadi H (2015) Computational study on ammonia adsorption on the X12Y12 nano-clusters (X = B, Al and Y = N, P). Curr Sci 108:1910–1914
Padash R, Rahimi-Nasrabadi M, Rad AS, Sobhani-Nasab A, Jesionowski T, Ehrlich H (2019) A comparative computational investigation of phosgene adsorption on (XY)12 (X = Al, B and Y = N, P) nanoclusters: DFT investigations. J Clust Sci 30:203–218. https://doi.org/10.1007/s10876-018-1479-y
Noei M (2017) Different electronic sensitivity of BN and AlN nanoclusters to SO2 gas: DFT studies. Vacuum 135:44–49. https://doi.org/10.1016/j.vacuum.2016.10.029
Baei MT, Ghasemi AS, Lemeski ET, Soltani A, Gholami N (2016) BN nanotube serving as a gas chemical sensor for N2O by parallel electric field. J Clust Sci 27:1081–1096. https://doi.org/10.1007/s10876-016-0969-z
Farrokhpour H, Jouypazadeh H, Sohroforouzani SV (2020) Interaction of different types of nanocages (Al12N12, Al12P12, B12N12, Be12O12, Mg12O12, Si12C12 and C24) with HCN and ClCN: DFT, TD-DFT, QTAIM, and NBO calculations. Mol Phys 118:e1626506. https://doi.org/10.1080/00268976.2019.1626506
Rad AS (2017) Application of B12N12 and B12P12 as two fullerene-like semiconductors for adsorption of halomethane: density functional theory study. Semiconductors 51:134–138. https://doi.org/10.1134/S1063782617010225
Mohammadi MD, Abdullah HY, Kalamse VG, Chaudhari A (2022) Interaction of halomethane CH3Z (Z = F, Cl, Br) with X12Y12 (X = B, Al, Ga & Y = N, P, As) nanocages. Comput Theor Chem 1208:113544. https://doi.org/10.1016/j.comptc.2021.113544
Soltani A, Javan MB (2015) Carbon monoxide interactions with pure and doped B11XN12 (X = Mg, Ge, Ga) nano-clusters: a theoretical study. RSC Adv 5:90621–90631. https://doi.org/10.1039/C5RA12571E
Solimannejad M, Kamalinahad S, Shakerzadeh E (2016) Sensing performance of Sc-doped B12N12 nanocage for detecting toxic cyanogen gas: a computational study. Phys Chem Res 4:315–332. https://doi.org/10.22036/pcr.2016.14142
Rahimi F, Zabaradsti A (2017) Photo-induced electron transfer process on pristine and Sc-substituted B12N12 nanocage as H2S chemosensor: a fully DFT and TD-DFT study. J Inorg Organomet Polym 27:1770–1777. https://doi.org/10.1007/s10904-017-0640-7
Shakerzadeh E, Khodayar E, Noorizadeh S (2016) Theoretical assessment of phosgene adsorption behavior onto pristine, Al- and Ga-doped B12N12 and B16N16 nanoclusters. Comput Mater Sci 118:155–171. https://doi.org/10.1016/j.commatsci.2016.03.016
Yong Y, Jiang H, Li X, Lv S, Cao J (2016) The cluster-assembled nanowires based on M12N12 (M = Al and Ga) clusters as potential gas sensors for CO, NO, and NO2 detection. Phys Chem Chem Phys 18:21431–21441. https://doi.org/10.1039/C6CP02931K
Kaewmaraya T, Ngamwongwan L, Moontragoon P, Jarernboon W, Singh D, Ahuja R, Karton A, Hussain T (2021) Novel green phosphorene as a superior chemical gas sensing material. J Hazard Mater 401:123340. https://doi.org/10.1016/j.jhazmat.2020.123340
Rad AS, Ayub K (2017) O3 and SO2 sensing concept on extended surface of B12N12 nanocages modified by Nickel decoration: a comprehensive DFT study. Solid State Sci 69:22–30. https://doi.org/10.1016/j.solidstatesciences.2017.05.007
Larki S, Shakerzadeh E, Anota EC, Behjatmanesh-Ardakani R (2019) The Al, Ga and Sc dopants effect on the adsorption performance of B12N12 nanocluster toward pnictogen hydrides. Chem Phys 526:110424. https://doi.org/10.1016/j.chemphys.2019.110424
Azimi F, Tazikeh-Lemeski E (2020) Exploring adsorption behavior of cyanogen chloride molecule on boron nitride nanocluster from first-principles. Russ J Phys Chem A 94:2141–2147. https://doi.org/10.1134/S0036024420100039
Ammar HY, Eid KhM, Badran HM (2020) Interaction and detection of formaldehyde on pristine and doped boron nitride nano-cage: DFT calculations. Mater Today Commun 25:101408. https://doi.org/10.1016/j.mtcomm.2020.101408
Hussain S, Hussain R, Mehboob MY, Chatha SAS, Hussain AI, Umar A, Khan MU, Ahmed M, Adnan M, Ayub K (2020) Adsorption of phosgene gas on pristine and copper-decorated B12N12 nanocages: a comparative DFT study. ACS Omega 5:7641–7650. https://doi.org/10.1021/acsomega.0c00507
Ammar HY, Badran HM, Eid KhM (2020) TM-doped B12N12 nano-cage (TM = Mn, Fe) as a sensor for CO, NO, and NH3 gases: a DFT and TD-DFT study. Mater Today Commun 25:101681. https://doi.org/10.1016/j.mtcomm.2020.101681
Acknowledgements
The authors are grateful for the financial support provided by CAPES, CNPq, and FAPEMA.
Funding
This work is supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—grant No. 88887.472618/2019–00-PROCAD-AM).
Author information
Authors and Affiliations
Contributions
Adilson L. P. Silva: investigation, writing, reviewing, and editing. Natanael de S. Sousa: investigation, writing. Jaldyr de J. G. Varela Júnior: conceptualization, methodology, resources, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, A.L.P., de Sousa Sousa, N. & de Jesus Gomes Varela Júnior, J. Theoretical studies with B12N12 as a toxic gas sensor: a review. J Nanopart Res 25, 22 (2023). https://doi.org/10.1007/s11051-023-05667-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05667-9